Circulating Specialized Proresolving Lipid Mediators after Short-term EPA/DHA-Supplementation
This article at a glance  At this stage it is not yet clear whether these substances do really play a role in humans, since studies reporting the measurement of SPMs in humans are few, and not much knowledge has been gained yet on which SPMs are formed under what conditions in healthy persons or those with specific disorders. From animal models we estimate that the body employs several different counter-regulatory mediators in order to be able to resolve different pathological processes, using combinations of SPMs that are produced in a temporally-defined order. Many types of tissue injury can of course happen, and a superimposed infection with one of many pathogens is a further possible event. At present several research groups have started staking out the terrain to identify which SPMs can be detected in humans under which conditions. Since the concentrations of SPMs in vivo are generally very low (picomolar to low nanomolar) and metabolic inactivation is considered to happen fast, technical challenges exist in reliably extracting and measuring SPMs. Autacoids are typically formed in a cell-specific fashion, act locally on receptors in neighboring cells, and are thereafter degraded rapidly. SPM levels depend on a combination of local conditions that determine substrate availability, and the expression and activities of enzymes that are involved in the biosynthesis and degradation of specific SPMs. Since autacoids act locally, it is currently not clear whether they can be detected in the circulation, and whether there might even exist a role for circulating SPMs.
At this stage it is not yet clear whether these substances do really play a role in humans, since studies reporting the measurement of SPMs in humans are few, and not much knowledge has been gained yet on which SPMs are formed under what conditions in healthy persons or those with specific disorders. From animal models we estimate that the body employs several different counter-regulatory mediators in order to be able to resolve different pathological processes, using combinations of SPMs that are produced in a temporally-defined order. Many types of tissue injury can of course happen, and a superimposed infection with one of many pathogens is a further possible event. At present several research groups have started staking out the terrain to identify which SPMs can be detected in humans under which conditions. Since the concentrations of SPMs in vivo are generally very low (picomolar to low nanomolar) and metabolic inactivation is considered to happen fast, technical challenges exist in reliably extracting and measuring SPMs. Autacoids are typically formed in a cell-specific fashion, act locally on receptors in neighboring cells, and are thereafter degraded rapidly. SPM levels depend on a combination of local conditions that determine substrate availability, and the expression and activities of enzymes that are involved in the biosynthesis and degradation of specific SPMs. Since autacoids act locally, it is currently not clear whether they can be detected in the circulation, and whether there might even exist a role for circulating SPMs.  A new concept termed resolution pharmacology has emerged that aims to determine how pro-resolving substances can be employed to actively turn off inflammatory disease by stimulating resolution of inflammation. Of interest, the oldest synthetic drug we know, aspirin, was found already in the 1980s to trigger the enzymatic formation of so-called epi-lipoxins, derivatives of arachidonic acid (AA) with potent anti-inflammatory activity. Aspirin, while blocking prostanoid biosynthesis, can change the activity of the type-2 cyclooxygenase to an enzymatic activity that catalyzes the incorporation of oxygen into AA with a stereochemical orientation that was inverse (“epimeric”) to the commonly known stereochemistry of such reactions, typically carried out by another enzyme class called lipoxygenases. The epi-lipoxins proved to activate receptors on immune cells that turn off inflammation. From this perspective, aspirin is an unusual non-steroidal anti-inflammatory drug (NSAID) because it also actively triggers activation of anti-inflammation.
A new concept termed resolution pharmacology has emerged that aims to determine how pro-resolving substances can be employed to actively turn off inflammatory disease by stimulating resolution of inflammation. Of interest, the oldest synthetic drug we know, aspirin, was found already in the 1980s to trigger the enzymatic formation of so-called epi-lipoxins, derivatives of arachidonic acid (AA) with potent anti-inflammatory activity. Aspirin, while blocking prostanoid biosynthesis, can change the activity of the type-2 cyclooxygenase to an enzymatic activity that catalyzes the incorporation of oxygen into AA with a stereochemical orientation that was inverse (“epimeric”) to the commonly known stereochemistry of such reactions, typically carried out by another enzyme class called lipoxygenases. The epi-lipoxins proved to activate receptors on immune cells that turn off inflammation. From this perspective, aspirin is an unusual non-steroidal anti-inflammatory drug (NSAID) because it also actively triggers activation of anti-inflammation.  This idea of epimeric oxygenation was more recently confirmed to also operate with omega-3 LCPUFA, with the discovery of the first resolvin, resolvin E1 (RvE1). Here aspirin triggered the formation of 18R-hydroperoxy-ecosapentaenoic acid from EPA. This epimeric EPA derivative is then further oxygenated by a second enzyme (5-lipoxygenase) and undergoes structural rearrangement to form the trihydroxylated EPA derivative RvE1. However, non-epimeric resolvins were subsequently also shown to be formed, either by fatty acid oxygenases that incorporate oxygen with the “normal” S stereochemistry, or because cyclooxygenase can form both epimeric and non-epimeric forms. In the latter scenario, the epimeric forms are more easily measurable since they resist one specific route of metabolic degradation (dehydrogenation) better, resulting in the selective measurement of the epimeric forms. A range of SPMs are now known, with E-series resolvins being derived from EPA and D-series-resolvins, maresins and protectin D1 derived from DHA. For many SPMs, both S and R stereoisomers are now known to exist, depending on the route of enzymatic formation. Aspirin has shown us that modulation of SPM biosynthesis is a relevant concept for pharmacology. It has remained unclear to what extent epimeric SPMs may be formed preferentially after aspirin is taken by humans. It has also not yet been firmly established whether a short-term increase in the dietary intake of EPA and DHA will be mirrored in a change of the levels of SPMs in the circulation in humans. In order to address these questions, a recent study by Barden and colleagues from the School of Medicine and Pharmacology at the Royal Perth Hospital Unit, and the Western Australian Institute for Medical Research, University of Western Australia, in Perth, has carried out an intervention study in human volunteers to measure the circulating levels of several EPA- and DHA-derived mediators after supplementation with EPA/DHA. They also studied the effect of aspirin. Twenty-one healthy adult women and men (age 40-70 years) all received an EPA/DHA supplement for seven days. Each day, the study subjects ingested four capsules of EPA and DHA in the form of fish oil (triglycerides) amounting to a total dose of 1400 mg EPA and 1000 mg DHA. The capsules were taken throughout the day with meals. On day 5, the participants were randomly divided into two groups after matching for age and gender, and 11 persons started taking 300 mg enteric-coated aspirin for another two days. Aspirin was taken as three tablets of 100 mg, at breakfast, lunch and dinner. Blood samples were collected on day 0, day 5 and at day 7 (12 hours after the last dose of aspirin). Compliance with the omega-3 and aspirin dosing was nearly 100%.
This idea of epimeric oxygenation was more recently confirmed to also operate with omega-3 LCPUFA, with the discovery of the first resolvin, resolvin E1 (RvE1). Here aspirin triggered the formation of 18R-hydroperoxy-ecosapentaenoic acid from EPA. This epimeric EPA derivative is then further oxygenated by a second enzyme (5-lipoxygenase) and undergoes structural rearrangement to form the trihydroxylated EPA derivative RvE1. However, non-epimeric resolvins were subsequently also shown to be formed, either by fatty acid oxygenases that incorporate oxygen with the “normal” S stereochemistry, or because cyclooxygenase can form both epimeric and non-epimeric forms. In the latter scenario, the epimeric forms are more easily measurable since they resist one specific route of metabolic degradation (dehydrogenation) better, resulting in the selective measurement of the epimeric forms. A range of SPMs are now known, with E-series resolvins being derived from EPA and D-series-resolvins, maresins and protectin D1 derived from DHA. For many SPMs, both S and R stereoisomers are now known to exist, depending on the route of enzymatic formation. Aspirin has shown us that modulation of SPM biosynthesis is a relevant concept for pharmacology. It has remained unclear to what extent epimeric SPMs may be formed preferentially after aspirin is taken by humans. It has also not yet been firmly established whether a short-term increase in the dietary intake of EPA and DHA will be mirrored in a change of the levels of SPMs in the circulation in humans. In order to address these questions, a recent study by Barden and colleagues from the School of Medicine and Pharmacology at the Royal Perth Hospital Unit, and the Western Australian Institute for Medical Research, University of Western Australia, in Perth, has carried out an intervention study in human volunteers to measure the circulating levels of several EPA- and DHA-derived mediators after supplementation with EPA/DHA. They also studied the effect of aspirin. Twenty-one healthy adult women and men (age 40-70 years) all received an EPA/DHA supplement for seven days. Each day, the study subjects ingested four capsules of EPA and DHA in the form of fish oil (triglycerides) amounting to a total dose of 1400 mg EPA and 1000 mg DHA. The capsules were taken throughout the day with meals. On day 5, the participants were randomly divided into two groups after matching for age and gender, and 11 persons started taking 300 mg enteric-coated aspirin for another two days. Aspirin was taken as three tablets of 100 mg, at breakfast, lunch and dinner. Blood samples were collected on day 0, day 5 and at day 7 (12 hours after the last dose of aspirin). Compliance with the omega-3 and aspirin dosing was nearly 100%.  The research group that carried out this work is well known for its outstanding quantitative skills for measurement of SPMs in human plasma samples. The optimization of plasma preparation and extraction procedures was previously validated. Optimization of separation, ionization, fragmentation and detection has allowed reliable detection of small quantities of lipid mediators, making possible the measurement of very low concentrations of some SPMs in human EDTA plasma. SPMs were detected by multiple reaction monitoring by triple quadrupole mass spectrometry (MS) after separation of lipid mediators by liquid chromatography (LC). This study extended detection capabilities by implementing LC-based chiral separation prior to MS detection, allowing quantitative measurement of individual stereoisomers of a few select lipid mediators derived from EPA and DHA, namely the isomeric pairs 18R-HEPE (18R-hydroxy-eicosapentaenoic acid) and 18S-HEPE, RvE3 and 18R-RvE3, 17S-HDHA (17S-hydroxy-docosahexaenoic acid) and 17R-HDHA, RvD1 and 17R-RvD1, and 14S-HDHA (14S-hydroxy-docosahexaenoic acid) and 14R-HDHA. Analysis of the plasma samples revealed that several E-series resolvins (RvE1, RvE2, RvE3 and 18R-RvE3) and D-series resolvins (RvD1, 17R-RvD1 and RvD2) were present at measurable levels. Plasma concentrations were in the mid pg/ml range, ranging from approximately 25 pg/ml RvE3 to ~100 pg/ml RvE2. Also found were the singly oxygenated products 18R/S-HEPE (derived from EPA; about 90 pg/ml), 17R/S-HDHA (derived from DHA; about 80 pg/ml), and 14R/S-HDHA (derived from DHA) with higher levels reaching ~380 pg/ml. After five days of fish oil supplementation, significant increases in the plasma levels of 18R/S-HEPE, RvE1, 17R/S-HDHA, and 14R/S-HDHA were found. No changes in the concentrations of the other mediators were found. Two additional days of taking aspirin (300 mg/d) had no effect on the plasma levels of lipid mediators that had been observed at day 5 compared to the levels in control subjects not taking aspirin. When the individual stereoisomers 18S-HEPE, 18R-HEPE, 17S-HDHA and 17R-HDHA were determined by chiral analysis, it was found that aspirin intake did not significantly change their plasma concentrations compared to controls. The ratio of 17R-HDHA to 17S-HDHA plasma concentrations decreased at day 7 compared to day 5 in controls. In aspirin-treated subjects the ratio was maintained and was significantly higher than in control subjects. The ratio of R- and S-isomers of 18-HEPE, RvE3 and RvD1 did not change after aspirin treatment.
The research group that carried out this work is well known for its outstanding quantitative skills for measurement of SPMs in human plasma samples. The optimization of plasma preparation and extraction procedures was previously validated. Optimization of separation, ionization, fragmentation and detection has allowed reliable detection of small quantities of lipid mediators, making possible the measurement of very low concentrations of some SPMs in human EDTA plasma. SPMs were detected by multiple reaction monitoring by triple quadrupole mass spectrometry (MS) after separation of lipid mediators by liquid chromatography (LC). This study extended detection capabilities by implementing LC-based chiral separation prior to MS detection, allowing quantitative measurement of individual stereoisomers of a few select lipid mediators derived from EPA and DHA, namely the isomeric pairs 18R-HEPE (18R-hydroxy-eicosapentaenoic acid) and 18S-HEPE, RvE3 and 18R-RvE3, 17S-HDHA (17S-hydroxy-docosahexaenoic acid) and 17R-HDHA, RvD1 and 17R-RvD1, and 14S-HDHA (14S-hydroxy-docosahexaenoic acid) and 14R-HDHA. Analysis of the plasma samples revealed that several E-series resolvins (RvE1, RvE2, RvE3 and 18R-RvE3) and D-series resolvins (RvD1, 17R-RvD1 and RvD2) were present at measurable levels. Plasma concentrations were in the mid pg/ml range, ranging from approximately 25 pg/ml RvE3 to ~100 pg/ml RvE2. Also found were the singly oxygenated products 18R/S-HEPE (derived from EPA; about 90 pg/ml), 17R/S-HDHA (derived from DHA; about 80 pg/ml), and 14R/S-HDHA (derived from DHA) with higher levels reaching ~380 pg/ml. After five days of fish oil supplementation, significant increases in the plasma levels of 18R/S-HEPE, RvE1, 17R/S-HDHA, and 14R/S-HDHA were found. No changes in the concentrations of the other mediators were found. Two additional days of taking aspirin (300 mg/d) had no effect on the plasma levels of lipid mediators that had been observed at day 5 compared to the levels in control subjects not taking aspirin. When the individual stereoisomers 18S-HEPE, 18R-HEPE, 17S-HDHA and 17R-HDHA were determined by chiral analysis, it was found that aspirin intake did not significantly change their plasma concentrations compared to controls. The ratio of 17R-HDHA to 17S-HDHA plasma concentrations decreased at day 7 compared to day 5 in controls. In aspirin-treated subjects the ratio was maintained and was significantly higher than in control subjects. The ratio of R- and S-isomers of 18-HEPE, RvE3 and RvD1 did not change after aspirin treatment.  The study is important as it expands our understanding of the baseline levels of EPA- and DHA-derived SPMs in healthy adults, and how these levels change after a relatively short term increase in omega-3 LCPUFA intake from fish oil. This study confirms the presence of RvD1 and RvD2 in the plasma of healthy human volunteers. Interestingly, the study has been able to detect resolvin E3 (RvE3), the most recently discovered E-series resolvin (Figure 1). No significant changes in RvE3 levels were detected upon EPA/DHA supplementation or the aspirin challenge. The study has also been able to detect RvE1 in plasma, an E-series resolvin known to be rapidly metabolized and that easily escapes detection. In a previous report RvE1 was not detected in plasma, and further optimization of sample preparation conditions with the reported inclusion of glutathione and BHT may have aided in this respect. Furthermore, the 5-day supplementation with EPA/DHA resulted in a significant increase in the plasma levels of RvE1. At present, it is hard to define the origin of the various SPMs detected in plasma. It is well possible that SPMs found in plasma are not a reflection of their formation in specific organs, since tissue levels are likely to be tightly controlled and SPMs may or may not diffuse into the circulation. Their presence in plasma may be a reflection of formation by circulating blood cells and platelets, as well as by the vascular endothelium. This study now makes possible further investigations to determine SPM levels occurring in the context of different inflammatory pathologies, allowing the understanding to what extent increased SPM biosynthesis will be detectable in the circulation.
The study is important as it expands our understanding of the baseline levels of EPA- and DHA-derived SPMs in healthy adults, and how these levels change after a relatively short term increase in omega-3 LCPUFA intake from fish oil. This study confirms the presence of RvD1 and RvD2 in the plasma of healthy human volunteers. Interestingly, the study has been able to detect resolvin E3 (RvE3), the most recently discovered E-series resolvin (Figure 1). No significant changes in RvE3 levels were detected upon EPA/DHA supplementation or the aspirin challenge. The study has also been able to detect RvE1 in plasma, an E-series resolvin known to be rapidly metabolized and that easily escapes detection. In a previous report RvE1 was not detected in plasma, and further optimization of sample preparation conditions with the reported inclusion of glutathione and BHT may have aided in this respect. Furthermore, the 5-day supplementation with EPA/DHA resulted in a significant increase in the plasma levels of RvE1. At present, it is hard to define the origin of the various SPMs detected in plasma. It is well possible that SPMs found in plasma are not a reflection of their formation in specific organs, since tissue levels are likely to be tightly controlled and SPMs may or may not diffuse into the circulation. Their presence in plasma may be a reflection of formation by circulating blood cells and platelets, as well as by the vascular endothelium. This study now makes possible further investigations to determine SPM levels occurring in the context of different inflammatory pathologies, allowing the understanding to what extent increased SPM biosynthesis will be detectable in the circulation. 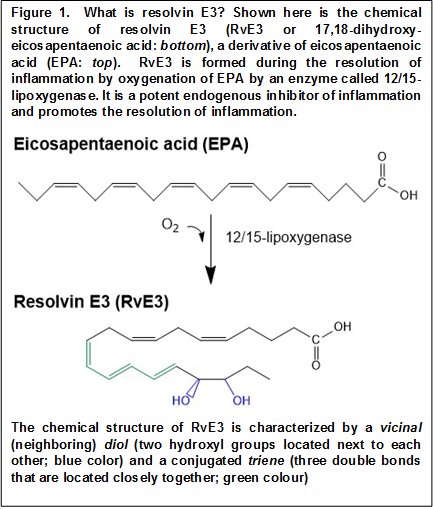 The alterations in the levels of RvE1 and the monohydroxylated precursors 18-HEPE, 14-HDHA and 17-HDHA following changes in EPA and DHA status observed in the present study are suggestive of blood acting as a medium that carries and distributes anti-inflammatory lipid mediators. The failure to observe increases in most other SPMs besides RvE1 after EPA/DHA supplementation also indicates that systemic elimination of select SPMs may take place, for example through pulmonary dehydrogenation, keeping baseline levels within a tight range irrespective of substrate availability. Why RvE1 is sensitive to omega-3 supplementation in the healthy state will be an interesting topic to explore further. But as the prime example of an epimeric resolvin, expansion of chiral analysis to additional lipid mediators (including 18S-RvE1) will likely inform us further concerning the importance of selective metabolic stability and probable longer-lasting endogenous actions of aspirin-triggered SPMs for endogenous inflammation control. The lack of effect of a 3x100 mg daily aspirin dose for two days on the plasma levels of the measured lipid mediators indicates that their circulating levels may be determined by substrate availability but not by aspirin-triggered SPM biosynthesis within tissues. The study shows that the changes in epimeric oxygenation triggered by aspirin, as reported in cellular systems, are difficult to appreciate from the circulating levels of SPMs and their singly-oxygenated precursors. Only for 17-HDHA has evidence has been gleaned that aspirin changes the relative formation of the two stereoisomers. In any case, the relative presence of 17R-HDHA decreased compared to 17S-HDHA, which is not supportive of an aspirin-triggered effect on cyclooxygenase-2 in this setting. This intervention study was performed with healthy volunteers that have no overt inflammation, and any involvement of a constitutively expressed cyclooxygenase-2, e.g. in the kidney, would have been expected to have resulted in an opposite change in ratio. Aspirin did not significantly change the levels of RvE1 in plasma, even though RvE1 is considered the archetypal “aspirin-triggered” omega-3 LCPUFA-derived SPM. This observation suggests that RvE1 found in plasma does not involve an enzymatic source that can be further activated by aspirin, such as cyclooxygenase-2, or cytochrome P450 in the liver during first-pass metabolism of aspirin. Taken together, at present, measurements of plasma levels of stereoisomers of select SPMs do not provide evidence for aspirin-triggered modulation of SPM biosynthesis in healthy adults. However, the results should not be taken as evidence for the absence of aspirin-triggered resolvin formation within tissues at present.
The alterations in the levels of RvE1 and the monohydroxylated precursors 18-HEPE, 14-HDHA and 17-HDHA following changes in EPA and DHA status observed in the present study are suggestive of blood acting as a medium that carries and distributes anti-inflammatory lipid mediators. The failure to observe increases in most other SPMs besides RvE1 after EPA/DHA supplementation also indicates that systemic elimination of select SPMs may take place, for example through pulmonary dehydrogenation, keeping baseline levels within a tight range irrespective of substrate availability. Why RvE1 is sensitive to omega-3 supplementation in the healthy state will be an interesting topic to explore further. But as the prime example of an epimeric resolvin, expansion of chiral analysis to additional lipid mediators (including 18S-RvE1) will likely inform us further concerning the importance of selective metabolic stability and probable longer-lasting endogenous actions of aspirin-triggered SPMs for endogenous inflammation control. The lack of effect of a 3x100 mg daily aspirin dose for two days on the plasma levels of the measured lipid mediators indicates that their circulating levels may be determined by substrate availability but not by aspirin-triggered SPM biosynthesis within tissues. The study shows that the changes in epimeric oxygenation triggered by aspirin, as reported in cellular systems, are difficult to appreciate from the circulating levels of SPMs and their singly-oxygenated precursors. Only for 17-HDHA has evidence has been gleaned that aspirin changes the relative formation of the two stereoisomers. In any case, the relative presence of 17R-HDHA decreased compared to 17S-HDHA, which is not supportive of an aspirin-triggered effect on cyclooxygenase-2 in this setting. This intervention study was performed with healthy volunteers that have no overt inflammation, and any involvement of a constitutively expressed cyclooxygenase-2, e.g. in the kidney, would have been expected to have resulted in an opposite change in ratio. Aspirin did not significantly change the levels of RvE1 in plasma, even though RvE1 is considered the archetypal “aspirin-triggered” omega-3 LCPUFA-derived SPM. This observation suggests that RvE1 found in plasma does not involve an enzymatic source that can be further activated by aspirin, such as cyclooxygenase-2, or cytochrome P450 in the liver during first-pass metabolism of aspirin. Taken together, at present, measurements of plasma levels of stereoisomers of select SPMs do not provide evidence for aspirin-triggered modulation of SPM biosynthesis in healthy adults. However, the results should not be taken as evidence for the absence of aspirin-triggered resolvin formation within tissues at present.  It is interesting to note that 14R/S-HDHA was present in plasma at relatively high levels. 14R/S-HDHA is a DHA-derivative that may be taken as a gauge for biosynthetic capacity of the formation of maresins, which are macrophage and platelet-derived SPMs. At present, it is not possible to deduce whether higher plasma levels of 14-HDHA mean that maresin biosynthesis is more active in healthy adults than other biosynthetic routes, that 14-HDHA is relatively more resistant from metabolic inactivation, or that 14-HDHA represents an accumulation of singly oxygenated and reduced product from DHA that is not further oxygenated to maresins. Taken together, this study has again advanced our limits of understanding on the involvement of omega-3 LCPUFA in human physiology by providing a proper description of EPA-and DHA-derived SPMs in the circulation. With this knowledge subsequent studies will have a suitable reference, undoubtedly surprising us with more insight into the relationship between essential acid sufficiency and the bioactivity of their downstream mediators. Barden A, Mas E, Croft KD, Phillips M, Mori TA. Short-term n-3 fatty acid supplementation but not aspirin increases plasma proresolving mediators of inflammation. J. Lipid Res. 2014;55(11):2401-2407. [PubMed] Worth Noting Abdulnour RE, Dalli J, Colby JK, Krishnamoorthy N, Timmons JY, Tan SH, Colas RA, Petasis NA, Serhan CN, Levy BD. Maresin 1 biosynthesis during platelet-neutrophil interactions is organ-protective. Proc. Natl. Acad. Sci. U.S.A. 2014;111(46):16526-16531. [PubMed] Eling TE, Ally AI. Pulmonary biosynthesis and metabolism of prostaglandins and related substances. Environ. Health Perspect. 1984;55:159-168. [PubMed] Isobe Y, Arita M, Matsueda S, Iwamoto R, Fujihara T, Nakanishi H, Taguchi R, Masuda K, Sasaki K, Urabe D, Inoue M, Arai H. Identification and structural determination of novel anti-inflammatory mediator resolvin E3, 17,18-dihydroxyeicosapentaenoic acid. J. Biol. Chem. 2012;287(13):10525-10534. [PubMed] Mas E, Croft KD, Zahra P, Barden A, Mori TA. Resolvins D1, D2, and other mediators of self-limited resolution of inflammation in human blood following n-3 fatty acid supplementation. Clin. Chem. 2012;58(10):1476-1484. [PubMed] Serhan CN, Chiang N, Dalli J, Levy BD. Lipid Mediators in the resolution of inflammation. Cold Spring Harb. Perspect. Biol. 2014;7(2). [PubMed] Titos E, Chiang N, Serhan CN, Romano M, Gaya J, Pueyo G, Clària J. Hepatocytes are a rich source of novel aspirin-triggered 15-epi-lipoxin A4. Am. J. Physiol. 1999;277(5 Pt 1):C870-877. [PubMed]
It is interesting to note that 14R/S-HDHA was present in plasma at relatively high levels. 14R/S-HDHA is a DHA-derivative that may be taken as a gauge for biosynthetic capacity of the formation of maresins, which are macrophage and platelet-derived SPMs. At present, it is not possible to deduce whether higher plasma levels of 14-HDHA mean that maresin biosynthesis is more active in healthy adults than other biosynthetic routes, that 14-HDHA is relatively more resistant from metabolic inactivation, or that 14-HDHA represents an accumulation of singly oxygenated and reduced product from DHA that is not further oxygenated to maresins. Taken together, this study has again advanced our limits of understanding on the involvement of omega-3 LCPUFA in human physiology by providing a proper description of EPA-and DHA-derived SPMs in the circulation. With this knowledge subsequent studies will have a suitable reference, undoubtedly surprising us with more insight into the relationship between essential acid sufficiency and the bioactivity of their downstream mediators. Barden A, Mas E, Croft KD, Phillips M, Mori TA. Short-term n-3 fatty acid supplementation but not aspirin increases plasma proresolving mediators of inflammation. J. Lipid Res. 2014;55(11):2401-2407. [PubMed] Worth Noting Abdulnour RE, Dalli J, Colby JK, Krishnamoorthy N, Timmons JY, Tan SH, Colas RA, Petasis NA, Serhan CN, Levy BD. Maresin 1 biosynthesis during platelet-neutrophil interactions is organ-protective. Proc. Natl. Acad. Sci. U.S.A. 2014;111(46):16526-16531. [PubMed] Eling TE, Ally AI. Pulmonary biosynthesis and metabolism of prostaglandins and related substances. Environ. Health Perspect. 1984;55:159-168. [PubMed] Isobe Y, Arita M, Matsueda S, Iwamoto R, Fujihara T, Nakanishi H, Taguchi R, Masuda K, Sasaki K, Urabe D, Inoue M, Arai H. Identification and structural determination of novel anti-inflammatory mediator resolvin E3, 17,18-dihydroxyeicosapentaenoic acid. J. Biol. Chem. 2012;287(13):10525-10534. [PubMed] Mas E, Croft KD, Zahra P, Barden A, Mori TA. Resolvins D1, D2, and other mediators of self-limited resolution of inflammation in human blood following n-3 fatty acid supplementation. Clin. Chem. 2012;58(10):1476-1484. [PubMed] Serhan CN, Chiang N, Dalli J, Levy BD. Lipid Mediators in the resolution of inflammation. Cold Spring Harb. Perspect. Biol. 2014;7(2). [PubMed] Titos E, Chiang N, Serhan CN, Romano M, Gaya J, Pueyo G, Clària J. Hepatocytes are a rich source of novel aspirin-triggered 15-epi-lipoxin A4. Am. J. Physiol. 1999;277(5 Pt 1):C870-877. [PubMed]
- This study addressed the levels of anti-inflammatory and resolution-promoting lipid mediators derived from EPA and DHA in human plasma during oral supplementation with EPA/DHA over a relatively short period of five days.
- The potential effect of aspirin on the selective formation of stereoisomers of several derivatives was also assessed.
- The results show that resolvin E1 in plasma is sensitive to supplementation, and that one of the most recently discovered resolvins, RvE3, is present in the circulation.
 At this stage it is not yet clear whether these substances do really play a role in humans, since studies reporting the measurement of SPMs in humans are few, and not much knowledge has been gained yet on which SPMs are formed under what conditions in healthy persons or those with specific disorders. From animal models we estimate that the body employs several different counter-regulatory mediators in order to be able to resolve different pathological processes, using combinations of SPMs that are produced in a temporally-defined order. Many types of tissue injury can of course happen, and a superimposed infection with one of many pathogens is a further possible event. At present several research groups have started staking out the terrain to identify which SPMs can be detected in humans under which conditions. Since the concentrations of SPMs in vivo are generally very low (picomolar to low nanomolar) and metabolic inactivation is considered to happen fast, technical challenges exist in reliably extracting and measuring SPMs. Autacoids are typically formed in a cell-specific fashion, act locally on receptors in neighboring cells, and are thereafter degraded rapidly. SPM levels depend on a combination of local conditions that determine substrate availability, and the expression and activities of enzymes that are involved in the biosynthesis and degradation of specific SPMs. Since autacoids act locally, it is currently not clear whether they can be detected in the circulation, and whether there might even exist a role for circulating SPMs.
At this stage it is not yet clear whether these substances do really play a role in humans, since studies reporting the measurement of SPMs in humans are few, and not much knowledge has been gained yet on which SPMs are formed under what conditions in healthy persons or those with specific disorders. From animal models we estimate that the body employs several different counter-regulatory mediators in order to be able to resolve different pathological processes, using combinations of SPMs that are produced in a temporally-defined order. Many types of tissue injury can of course happen, and a superimposed infection with one of many pathogens is a further possible event. At present several research groups have started staking out the terrain to identify which SPMs can be detected in humans under which conditions. Since the concentrations of SPMs in vivo are generally very low (picomolar to low nanomolar) and metabolic inactivation is considered to happen fast, technical challenges exist in reliably extracting and measuring SPMs. Autacoids are typically formed in a cell-specific fashion, act locally on receptors in neighboring cells, and are thereafter degraded rapidly. SPM levels depend on a combination of local conditions that determine substrate availability, and the expression and activities of enzymes that are involved in the biosynthesis and degradation of specific SPMs. Since autacoids act locally, it is currently not clear whether they can be detected in the circulation, and whether there might even exist a role for circulating SPMs.  A new concept termed resolution pharmacology has emerged that aims to determine how pro-resolving substances can be employed to actively turn off inflammatory disease by stimulating resolution of inflammation. Of interest, the oldest synthetic drug we know, aspirin, was found already in the 1980s to trigger the enzymatic formation of so-called epi-lipoxins, derivatives of arachidonic acid (AA) with potent anti-inflammatory activity. Aspirin, while blocking prostanoid biosynthesis, can change the activity of the type-2 cyclooxygenase to an enzymatic activity that catalyzes the incorporation of oxygen into AA with a stereochemical orientation that was inverse (“epimeric”) to the commonly known stereochemistry of such reactions, typically carried out by another enzyme class called lipoxygenases. The epi-lipoxins proved to activate receptors on immune cells that turn off inflammation. From this perspective, aspirin is an unusual non-steroidal anti-inflammatory drug (NSAID) because it also actively triggers activation of anti-inflammation.
A new concept termed resolution pharmacology has emerged that aims to determine how pro-resolving substances can be employed to actively turn off inflammatory disease by stimulating resolution of inflammation. Of interest, the oldest synthetic drug we know, aspirin, was found already in the 1980s to trigger the enzymatic formation of so-called epi-lipoxins, derivatives of arachidonic acid (AA) with potent anti-inflammatory activity. Aspirin, while blocking prostanoid biosynthesis, can change the activity of the type-2 cyclooxygenase to an enzymatic activity that catalyzes the incorporation of oxygen into AA with a stereochemical orientation that was inverse (“epimeric”) to the commonly known stereochemistry of such reactions, typically carried out by another enzyme class called lipoxygenases. The epi-lipoxins proved to activate receptors on immune cells that turn off inflammation. From this perspective, aspirin is an unusual non-steroidal anti-inflammatory drug (NSAID) because it also actively triggers activation of anti-inflammation.  This idea of epimeric oxygenation was more recently confirmed to also operate with omega-3 LCPUFA, with the discovery of the first resolvin, resolvin E1 (RvE1). Here aspirin triggered the formation of 18R-hydroperoxy-ecosapentaenoic acid from EPA. This epimeric EPA derivative is then further oxygenated by a second enzyme (5-lipoxygenase) and undergoes structural rearrangement to form the trihydroxylated EPA derivative RvE1. However, non-epimeric resolvins were subsequently also shown to be formed, either by fatty acid oxygenases that incorporate oxygen with the “normal” S stereochemistry, or because cyclooxygenase can form both epimeric and non-epimeric forms. In the latter scenario, the epimeric forms are more easily measurable since they resist one specific route of metabolic degradation (dehydrogenation) better, resulting in the selective measurement of the epimeric forms. A range of SPMs are now known, with E-series resolvins being derived from EPA and D-series-resolvins, maresins and protectin D1 derived from DHA. For many SPMs, both S and R stereoisomers are now known to exist, depending on the route of enzymatic formation. Aspirin has shown us that modulation of SPM biosynthesis is a relevant concept for pharmacology. It has remained unclear to what extent epimeric SPMs may be formed preferentially after aspirin is taken by humans. It has also not yet been firmly established whether a short-term increase in the dietary intake of EPA and DHA will be mirrored in a change of the levels of SPMs in the circulation in humans. In order to address these questions, a recent study by Barden and colleagues from the School of Medicine and Pharmacology at the Royal Perth Hospital Unit, and the Western Australian Institute for Medical Research, University of Western Australia, in Perth, has carried out an intervention study in human volunteers to measure the circulating levels of several EPA- and DHA-derived mediators after supplementation with EPA/DHA. They also studied the effect of aspirin. Twenty-one healthy adult women and men (age 40-70 years) all received an EPA/DHA supplement for seven days. Each day, the study subjects ingested four capsules of EPA and DHA in the form of fish oil (triglycerides) amounting to a total dose of 1400 mg EPA and 1000 mg DHA. The capsules were taken throughout the day with meals. On day 5, the participants were randomly divided into two groups after matching for age and gender, and 11 persons started taking 300 mg enteric-coated aspirin for another two days. Aspirin was taken as three tablets of 100 mg, at breakfast, lunch and dinner. Blood samples were collected on day 0, day 5 and at day 7 (12 hours after the last dose of aspirin). Compliance with the omega-3 and aspirin dosing was nearly 100%.
This idea of epimeric oxygenation was more recently confirmed to also operate with omega-3 LCPUFA, with the discovery of the first resolvin, resolvin E1 (RvE1). Here aspirin triggered the formation of 18R-hydroperoxy-ecosapentaenoic acid from EPA. This epimeric EPA derivative is then further oxygenated by a second enzyme (5-lipoxygenase) and undergoes structural rearrangement to form the trihydroxylated EPA derivative RvE1. However, non-epimeric resolvins were subsequently also shown to be formed, either by fatty acid oxygenases that incorporate oxygen with the “normal” S stereochemistry, or because cyclooxygenase can form both epimeric and non-epimeric forms. In the latter scenario, the epimeric forms are more easily measurable since they resist one specific route of metabolic degradation (dehydrogenation) better, resulting in the selective measurement of the epimeric forms. A range of SPMs are now known, with E-series resolvins being derived from EPA and D-series-resolvins, maresins and protectin D1 derived from DHA. For many SPMs, both S and R stereoisomers are now known to exist, depending on the route of enzymatic formation. Aspirin has shown us that modulation of SPM biosynthesis is a relevant concept for pharmacology. It has remained unclear to what extent epimeric SPMs may be formed preferentially after aspirin is taken by humans. It has also not yet been firmly established whether a short-term increase in the dietary intake of EPA and DHA will be mirrored in a change of the levels of SPMs in the circulation in humans. In order to address these questions, a recent study by Barden and colleagues from the School of Medicine and Pharmacology at the Royal Perth Hospital Unit, and the Western Australian Institute for Medical Research, University of Western Australia, in Perth, has carried out an intervention study in human volunteers to measure the circulating levels of several EPA- and DHA-derived mediators after supplementation with EPA/DHA. They also studied the effect of aspirin. Twenty-one healthy adult women and men (age 40-70 years) all received an EPA/DHA supplement for seven days. Each day, the study subjects ingested four capsules of EPA and DHA in the form of fish oil (triglycerides) amounting to a total dose of 1400 mg EPA and 1000 mg DHA. The capsules were taken throughout the day with meals. On day 5, the participants were randomly divided into two groups after matching for age and gender, and 11 persons started taking 300 mg enteric-coated aspirin for another two days. Aspirin was taken as three tablets of 100 mg, at breakfast, lunch and dinner. Blood samples were collected on day 0, day 5 and at day 7 (12 hours after the last dose of aspirin). Compliance with the omega-3 and aspirin dosing was nearly 100%.  The research group that carried out this work is well known for its outstanding quantitative skills for measurement of SPMs in human plasma samples. The optimization of plasma preparation and extraction procedures was previously validated. Optimization of separation, ionization, fragmentation and detection has allowed reliable detection of small quantities of lipid mediators, making possible the measurement of very low concentrations of some SPMs in human EDTA plasma. SPMs were detected by multiple reaction monitoring by triple quadrupole mass spectrometry (MS) after separation of lipid mediators by liquid chromatography (LC). This study extended detection capabilities by implementing LC-based chiral separation prior to MS detection, allowing quantitative measurement of individual stereoisomers of a few select lipid mediators derived from EPA and DHA, namely the isomeric pairs 18R-HEPE (18R-hydroxy-eicosapentaenoic acid) and 18S-HEPE, RvE3 and 18R-RvE3, 17S-HDHA (17S-hydroxy-docosahexaenoic acid) and 17R-HDHA, RvD1 and 17R-RvD1, and 14S-HDHA (14S-hydroxy-docosahexaenoic acid) and 14R-HDHA. Analysis of the plasma samples revealed that several E-series resolvins (RvE1, RvE2, RvE3 and 18R-RvE3) and D-series resolvins (RvD1, 17R-RvD1 and RvD2) were present at measurable levels. Plasma concentrations were in the mid pg/ml range, ranging from approximately 25 pg/ml RvE3 to ~100 pg/ml RvE2. Also found were the singly oxygenated products 18R/S-HEPE (derived from EPA; about 90 pg/ml), 17R/S-HDHA (derived from DHA; about 80 pg/ml), and 14R/S-HDHA (derived from DHA) with higher levels reaching ~380 pg/ml. After five days of fish oil supplementation, significant increases in the plasma levels of 18R/S-HEPE, RvE1, 17R/S-HDHA, and 14R/S-HDHA were found. No changes in the concentrations of the other mediators were found. Two additional days of taking aspirin (300 mg/d) had no effect on the plasma levels of lipid mediators that had been observed at day 5 compared to the levels in control subjects not taking aspirin. When the individual stereoisomers 18S-HEPE, 18R-HEPE, 17S-HDHA and 17R-HDHA were determined by chiral analysis, it was found that aspirin intake did not significantly change their plasma concentrations compared to controls. The ratio of 17R-HDHA to 17S-HDHA plasma concentrations decreased at day 7 compared to day 5 in controls. In aspirin-treated subjects the ratio was maintained and was significantly higher than in control subjects. The ratio of R- and S-isomers of 18-HEPE, RvE3 and RvD1 did not change after aspirin treatment.
The research group that carried out this work is well known for its outstanding quantitative skills for measurement of SPMs in human plasma samples. The optimization of plasma preparation and extraction procedures was previously validated. Optimization of separation, ionization, fragmentation and detection has allowed reliable detection of small quantities of lipid mediators, making possible the measurement of very low concentrations of some SPMs in human EDTA plasma. SPMs were detected by multiple reaction monitoring by triple quadrupole mass spectrometry (MS) after separation of lipid mediators by liquid chromatography (LC). This study extended detection capabilities by implementing LC-based chiral separation prior to MS detection, allowing quantitative measurement of individual stereoisomers of a few select lipid mediators derived from EPA and DHA, namely the isomeric pairs 18R-HEPE (18R-hydroxy-eicosapentaenoic acid) and 18S-HEPE, RvE3 and 18R-RvE3, 17S-HDHA (17S-hydroxy-docosahexaenoic acid) and 17R-HDHA, RvD1 and 17R-RvD1, and 14S-HDHA (14S-hydroxy-docosahexaenoic acid) and 14R-HDHA. Analysis of the plasma samples revealed that several E-series resolvins (RvE1, RvE2, RvE3 and 18R-RvE3) and D-series resolvins (RvD1, 17R-RvD1 and RvD2) were present at measurable levels. Plasma concentrations were in the mid pg/ml range, ranging from approximately 25 pg/ml RvE3 to ~100 pg/ml RvE2. Also found were the singly oxygenated products 18R/S-HEPE (derived from EPA; about 90 pg/ml), 17R/S-HDHA (derived from DHA; about 80 pg/ml), and 14R/S-HDHA (derived from DHA) with higher levels reaching ~380 pg/ml. After five days of fish oil supplementation, significant increases in the plasma levels of 18R/S-HEPE, RvE1, 17R/S-HDHA, and 14R/S-HDHA were found. No changes in the concentrations of the other mediators were found. Two additional days of taking aspirin (300 mg/d) had no effect on the plasma levels of lipid mediators that had been observed at day 5 compared to the levels in control subjects not taking aspirin. When the individual stereoisomers 18S-HEPE, 18R-HEPE, 17S-HDHA and 17R-HDHA were determined by chiral analysis, it was found that aspirin intake did not significantly change their plasma concentrations compared to controls. The ratio of 17R-HDHA to 17S-HDHA plasma concentrations decreased at day 7 compared to day 5 in controls. In aspirin-treated subjects the ratio was maintained and was significantly higher than in control subjects. The ratio of R- and S-isomers of 18-HEPE, RvE3 and RvD1 did not change after aspirin treatment.  The study is important as it expands our understanding of the baseline levels of EPA- and DHA-derived SPMs in healthy adults, and how these levels change after a relatively short term increase in omega-3 LCPUFA intake from fish oil. This study confirms the presence of RvD1 and RvD2 in the plasma of healthy human volunteers. Interestingly, the study has been able to detect resolvin E3 (RvE3), the most recently discovered E-series resolvin (Figure 1). No significant changes in RvE3 levels were detected upon EPA/DHA supplementation or the aspirin challenge. The study has also been able to detect RvE1 in plasma, an E-series resolvin known to be rapidly metabolized and that easily escapes detection. In a previous report RvE1 was not detected in plasma, and further optimization of sample preparation conditions with the reported inclusion of glutathione and BHT may have aided in this respect. Furthermore, the 5-day supplementation with EPA/DHA resulted in a significant increase in the plasma levels of RvE1. At present, it is hard to define the origin of the various SPMs detected in plasma. It is well possible that SPMs found in plasma are not a reflection of their formation in specific organs, since tissue levels are likely to be tightly controlled and SPMs may or may not diffuse into the circulation. Their presence in plasma may be a reflection of formation by circulating blood cells and platelets, as well as by the vascular endothelium. This study now makes possible further investigations to determine SPM levels occurring in the context of different inflammatory pathologies, allowing the understanding to what extent increased SPM biosynthesis will be detectable in the circulation.
The study is important as it expands our understanding of the baseline levels of EPA- and DHA-derived SPMs in healthy adults, and how these levels change after a relatively short term increase in omega-3 LCPUFA intake from fish oil. This study confirms the presence of RvD1 and RvD2 in the plasma of healthy human volunteers. Interestingly, the study has been able to detect resolvin E3 (RvE3), the most recently discovered E-series resolvin (Figure 1). No significant changes in RvE3 levels were detected upon EPA/DHA supplementation or the aspirin challenge. The study has also been able to detect RvE1 in plasma, an E-series resolvin known to be rapidly metabolized and that easily escapes detection. In a previous report RvE1 was not detected in plasma, and further optimization of sample preparation conditions with the reported inclusion of glutathione and BHT may have aided in this respect. Furthermore, the 5-day supplementation with EPA/DHA resulted in a significant increase in the plasma levels of RvE1. At present, it is hard to define the origin of the various SPMs detected in plasma. It is well possible that SPMs found in plasma are not a reflection of their formation in specific organs, since tissue levels are likely to be tightly controlled and SPMs may or may not diffuse into the circulation. Their presence in plasma may be a reflection of formation by circulating blood cells and platelets, as well as by the vascular endothelium. This study now makes possible further investigations to determine SPM levels occurring in the context of different inflammatory pathologies, allowing the understanding to what extent increased SPM biosynthesis will be detectable in the circulation.  The alterations in the levels of RvE1 and the monohydroxylated precursors 18-HEPE, 14-HDHA and 17-HDHA following changes in EPA and DHA status observed in the present study are suggestive of blood acting as a medium that carries and distributes anti-inflammatory lipid mediators. The failure to observe increases in most other SPMs besides RvE1 after EPA/DHA supplementation also indicates that systemic elimination of select SPMs may take place, for example through pulmonary dehydrogenation, keeping baseline levels within a tight range irrespective of substrate availability. Why RvE1 is sensitive to omega-3 supplementation in the healthy state will be an interesting topic to explore further. But as the prime example of an epimeric resolvin, expansion of chiral analysis to additional lipid mediators (including 18S-RvE1) will likely inform us further concerning the importance of selective metabolic stability and probable longer-lasting endogenous actions of aspirin-triggered SPMs for endogenous inflammation control. The lack of effect of a 3x100 mg daily aspirin dose for two days on the plasma levels of the measured lipid mediators indicates that their circulating levels may be determined by substrate availability but not by aspirin-triggered SPM biosynthesis within tissues. The study shows that the changes in epimeric oxygenation triggered by aspirin, as reported in cellular systems, are difficult to appreciate from the circulating levels of SPMs and their singly-oxygenated precursors. Only for 17-HDHA has evidence has been gleaned that aspirin changes the relative formation of the two stereoisomers. In any case, the relative presence of 17R-HDHA decreased compared to 17S-HDHA, which is not supportive of an aspirin-triggered effect on cyclooxygenase-2 in this setting. This intervention study was performed with healthy volunteers that have no overt inflammation, and any involvement of a constitutively expressed cyclooxygenase-2, e.g. in the kidney, would have been expected to have resulted in an opposite change in ratio. Aspirin did not significantly change the levels of RvE1 in plasma, even though RvE1 is considered the archetypal “aspirin-triggered” omega-3 LCPUFA-derived SPM. This observation suggests that RvE1 found in plasma does not involve an enzymatic source that can be further activated by aspirin, such as cyclooxygenase-2, or cytochrome P450 in the liver during first-pass metabolism of aspirin. Taken together, at present, measurements of plasma levels of stereoisomers of select SPMs do not provide evidence for aspirin-triggered modulation of SPM biosynthesis in healthy adults. However, the results should not be taken as evidence for the absence of aspirin-triggered resolvin formation within tissues at present.
The alterations in the levels of RvE1 and the monohydroxylated precursors 18-HEPE, 14-HDHA and 17-HDHA following changes in EPA and DHA status observed in the present study are suggestive of blood acting as a medium that carries and distributes anti-inflammatory lipid mediators. The failure to observe increases in most other SPMs besides RvE1 after EPA/DHA supplementation also indicates that systemic elimination of select SPMs may take place, for example through pulmonary dehydrogenation, keeping baseline levels within a tight range irrespective of substrate availability. Why RvE1 is sensitive to omega-3 supplementation in the healthy state will be an interesting topic to explore further. But as the prime example of an epimeric resolvin, expansion of chiral analysis to additional lipid mediators (including 18S-RvE1) will likely inform us further concerning the importance of selective metabolic stability and probable longer-lasting endogenous actions of aspirin-triggered SPMs for endogenous inflammation control. The lack of effect of a 3x100 mg daily aspirin dose for two days on the plasma levels of the measured lipid mediators indicates that their circulating levels may be determined by substrate availability but not by aspirin-triggered SPM biosynthesis within tissues. The study shows that the changes in epimeric oxygenation triggered by aspirin, as reported in cellular systems, are difficult to appreciate from the circulating levels of SPMs and their singly-oxygenated precursors. Only for 17-HDHA has evidence has been gleaned that aspirin changes the relative formation of the two stereoisomers. In any case, the relative presence of 17R-HDHA decreased compared to 17S-HDHA, which is not supportive of an aspirin-triggered effect on cyclooxygenase-2 in this setting. This intervention study was performed with healthy volunteers that have no overt inflammation, and any involvement of a constitutively expressed cyclooxygenase-2, e.g. in the kidney, would have been expected to have resulted in an opposite change in ratio. Aspirin did not significantly change the levels of RvE1 in plasma, even though RvE1 is considered the archetypal “aspirin-triggered” omega-3 LCPUFA-derived SPM. This observation suggests that RvE1 found in plasma does not involve an enzymatic source that can be further activated by aspirin, such as cyclooxygenase-2, or cytochrome P450 in the liver during first-pass metabolism of aspirin. Taken together, at present, measurements of plasma levels of stereoisomers of select SPMs do not provide evidence for aspirin-triggered modulation of SPM biosynthesis in healthy adults. However, the results should not be taken as evidence for the absence of aspirin-triggered resolvin formation within tissues at present.  It is interesting to note that 14R/S-HDHA was present in plasma at relatively high levels. 14R/S-HDHA is a DHA-derivative that may be taken as a gauge for biosynthetic capacity of the formation of maresins, which are macrophage and platelet-derived SPMs. At present, it is not possible to deduce whether higher plasma levels of 14-HDHA mean that maresin biosynthesis is more active in healthy adults than other biosynthetic routes, that 14-HDHA is relatively more resistant from metabolic inactivation, or that 14-HDHA represents an accumulation of singly oxygenated and reduced product from DHA that is not further oxygenated to maresins. Taken together, this study has again advanced our limits of understanding on the involvement of omega-3 LCPUFA in human physiology by providing a proper description of EPA-and DHA-derived SPMs in the circulation. With this knowledge subsequent studies will have a suitable reference, undoubtedly surprising us with more insight into the relationship between essential acid sufficiency and the bioactivity of their downstream mediators. Barden A, Mas E, Croft KD, Phillips M, Mori TA. Short-term n-3 fatty acid supplementation but not aspirin increases plasma proresolving mediators of inflammation. J. Lipid Res. 2014;55(11):2401-2407. [PubMed] Worth Noting Abdulnour RE, Dalli J, Colby JK, Krishnamoorthy N, Timmons JY, Tan SH, Colas RA, Petasis NA, Serhan CN, Levy BD. Maresin 1 biosynthesis during platelet-neutrophil interactions is organ-protective. Proc. Natl. Acad. Sci. U.S.A. 2014;111(46):16526-16531. [PubMed] Eling TE, Ally AI. Pulmonary biosynthesis and metabolism of prostaglandins and related substances. Environ. Health Perspect. 1984;55:159-168. [PubMed] Isobe Y, Arita M, Matsueda S, Iwamoto R, Fujihara T, Nakanishi H, Taguchi R, Masuda K, Sasaki K, Urabe D, Inoue M, Arai H. Identification and structural determination of novel anti-inflammatory mediator resolvin E3, 17,18-dihydroxyeicosapentaenoic acid. J. Biol. Chem. 2012;287(13):10525-10534. [PubMed] Mas E, Croft KD, Zahra P, Barden A, Mori TA. Resolvins D1, D2, and other mediators of self-limited resolution of inflammation in human blood following n-3 fatty acid supplementation. Clin. Chem. 2012;58(10):1476-1484. [PubMed] Serhan CN, Chiang N, Dalli J, Levy BD. Lipid Mediators in the resolution of inflammation. Cold Spring Harb. Perspect. Biol. 2014;7(2). [PubMed] Titos E, Chiang N, Serhan CN, Romano M, Gaya J, Pueyo G, Clària J. Hepatocytes are a rich source of novel aspirin-triggered 15-epi-lipoxin A4. Am. J. Physiol. 1999;277(5 Pt 1):C870-877. [PubMed]
It is interesting to note that 14R/S-HDHA was present in plasma at relatively high levels. 14R/S-HDHA is a DHA-derivative that may be taken as a gauge for biosynthetic capacity of the formation of maresins, which are macrophage and platelet-derived SPMs. At present, it is not possible to deduce whether higher plasma levels of 14-HDHA mean that maresin biosynthesis is more active in healthy adults than other biosynthetic routes, that 14-HDHA is relatively more resistant from metabolic inactivation, or that 14-HDHA represents an accumulation of singly oxygenated and reduced product from DHA that is not further oxygenated to maresins. Taken together, this study has again advanced our limits of understanding on the involvement of omega-3 LCPUFA in human physiology by providing a proper description of EPA-and DHA-derived SPMs in the circulation. With this knowledge subsequent studies will have a suitable reference, undoubtedly surprising us with more insight into the relationship between essential acid sufficiency and the bioactivity of their downstream mediators. Barden A, Mas E, Croft KD, Phillips M, Mori TA. Short-term n-3 fatty acid supplementation but not aspirin increases plasma proresolving mediators of inflammation. J. Lipid Res. 2014;55(11):2401-2407. [PubMed] Worth Noting Abdulnour RE, Dalli J, Colby JK, Krishnamoorthy N, Timmons JY, Tan SH, Colas RA, Petasis NA, Serhan CN, Levy BD. Maresin 1 biosynthesis during platelet-neutrophil interactions is organ-protective. Proc. Natl. Acad. Sci. U.S.A. 2014;111(46):16526-16531. [PubMed] Eling TE, Ally AI. Pulmonary biosynthesis and metabolism of prostaglandins and related substances. Environ. Health Perspect. 1984;55:159-168. [PubMed] Isobe Y, Arita M, Matsueda S, Iwamoto R, Fujihara T, Nakanishi H, Taguchi R, Masuda K, Sasaki K, Urabe D, Inoue M, Arai H. Identification and structural determination of novel anti-inflammatory mediator resolvin E3, 17,18-dihydroxyeicosapentaenoic acid. J. Biol. Chem. 2012;287(13):10525-10534. [PubMed] Mas E, Croft KD, Zahra P, Barden A, Mori TA. Resolvins D1, D2, and other mediators of self-limited resolution of inflammation in human blood following n-3 fatty acid supplementation. Clin. Chem. 2012;58(10):1476-1484. [PubMed] Serhan CN, Chiang N, Dalli J, Levy BD. Lipid Mediators in the resolution of inflammation. Cold Spring Harb. Perspect. Biol. 2014;7(2). [PubMed] Titos E, Chiang N, Serhan CN, Romano M, Gaya J, Pueyo G, Clària J. Hepatocytes are a rich source of novel aspirin-triggered 15-epi-lipoxin A4. Am. J. Physiol. 1999;277(5 Pt 1):C870-877. [PubMed]

