The Development of Chemoresistance: A New Immune Cell-Activating Mechanism Involving Uncommon Lipids that Mediate a Stem Cell Response to Genotoxicity
This article at a glance
- This study identifies the omega-3 polyunsaturated fatty acid hexadecatetraenoic acid (16:4 n-3) as a ligand of GPR120, a receptor studied for its role in regulating inflammation, energy metabolism and satiety.
- 16:4 n-3, previously identified as a fatty acid released by mesenchymal stem cells following the exposure to platinum-containing chemotherapeutic drugs, activated GPR120 in a specific population of macrophages in the spleen.
- GPR120-positive splenic macrophages activated by 16:4 n-3 produced a unique lysophospholipid, lysophosphatidylcholine with esterified nervonic acid (lysoPC(24:1)), which mediated a chemoresistance response to platinum-based chemotherapeutics.
- This study has further uncovered the components of a systemic cascade of signals involving some previously poorly known lipids, that are involved in the response to drugs that are genotoxic and/or induce cell-cycle arrest, providing a new basis for future improvements in chemotherapeutic efficacy.
 A large portion of patients with cancer treated with chemotherapeutic agents develop resistance to the used drugs, significantly lowering their efficacy over time and allowing the recurrence of tumor growth. Understanding the molecular mechanisms of chemoresistance is of importance as it may allow the development of improved antitumoral interventions that overcome tumor chemoresistance development, increasing the efficacy of cancer treatment. Resistance to chemotherapy can involve different mechanisms that are intrinsic to tumor cells, such as increased repair of drug-induced tumor DNA alterations, and diminished drug uptake and augmented efflux that reduce the level of a chemotherapeutic drug in tumor cells. Extrinsic mechanisms of resistance are those that occur outside malignant cells, such as immune system-mediated survival signals that permit neoplastic growth in the face of chemotherapy, or increased metabolic inactivation of chemotherapeutic drugs by augmented metabolism and excretion from the body. The signal for extrinsic mechanisms of chemoresistance may still originate from the tumor, but precisely how these are initiated is often obscure.
A large portion of patients with cancer treated with chemotherapeutic agents develop resistance to the used drugs, significantly lowering their efficacy over time and allowing the recurrence of tumor growth. Understanding the molecular mechanisms of chemoresistance is of importance as it may allow the development of improved antitumoral interventions that overcome tumor chemoresistance development, increasing the efficacy of cancer treatment. Resistance to chemotherapy can involve different mechanisms that are intrinsic to tumor cells, such as increased repair of drug-induced tumor DNA alterations, and diminished drug uptake and augmented efflux that reduce the level of a chemotherapeutic drug in tumor cells. Extrinsic mechanisms of resistance are those that occur outside malignant cells, such as immune system-mediated survival signals that permit neoplastic growth in the face of chemotherapy, or increased metabolic inactivation of chemotherapeutic drugs by augmented metabolism and excretion from the body. The signal for extrinsic mechanisms of chemoresistance may still originate from the tumor, but precisely how these are initiated is often obscure.
Cis-platin is a frequently employed chemotherapeutic drug that intercalates into the DNA structure and brings replication of dividing tumor cells to a halt. In the course of addressing the extrinsic mechanisms of chemoresistance to cis-platin, researchers at the Netherlands Cancer Institute in Amsterdam, and the University of Utrecht, The Netherlands, made a particular observation a few years ago. Mesenchymal stem cells (MSCs) were found to release two relatively poorly studied fatty acids in response to platinum-based drugs used in cancer chemotherapy. MSCs are progenitor cells that are capable of replication as undifferentiated cells (stem cell renewal) or differentiation into different tissue cells such as bone, cartilage, muscle and tendon, fat, and cells that make up the bone marrow. Interestingly, the two “platinum-induced fatty acids” (abbreviated PIFAs), mediated a chemoresistance response to cis-platin in a mouse model of aggressive tumor development following the experimental implantation with tumor cells.
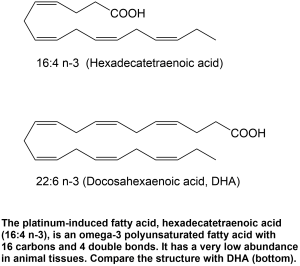 The two PIFA were identified as 12-oxo-heptadecatrienoic acid, and hexadecatetraenoic acid n-3 (16:4 n-3) (Fig 1). The former is a dehydrogenated derivative of 12-hydroxy-heptadecatrienoic acid (12-HHT), an arachidonic acid-derived lipid mediator formed during the enzymatic formation of thromboxane A2, a well-known vasoconstricting agent. 16:4 n-3 is a poorly documented omega-3 polyunsaturated fatty acid that is sometimes detected in animal tissues, but at far lower concentrations than the omega-3 PUFA alpha-linolenic acid (ALA), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA).
The two PIFA were identified as 12-oxo-heptadecatrienoic acid, and hexadecatetraenoic acid n-3 (16:4 n-3) (Fig 1). The former is a dehydrogenated derivative of 12-hydroxy-heptadecatrienoic acid (12-HHT), an arachidonic acid-derived lipid mediator formed during the enzymatic formation of thromboxane A2, a well-known vasoconstricting agent. 16:4 n-3 is a poorly documented omega-3 polyunsaturated fatty acid that is sometimes detected in animal tissues, but at far lower concentrations than the omega-3 PUFA alpha-linolenic acid (ALA), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA).
Since the initial report in 2011, the researchers have gradually carried out a characterization of the chemoresistance-inducing activity of PIFAs. PIFA did not affect the levels of cis-platin inside tumor cells, but did reduce the genotoxic (DNA-damaging) effect of cis-platin in tumor cells isolated from the mouse model. 16:4 n-3 acted in very low doses as a chemoresistance-inducing compound in mice with implanted tumor cells, but did not appear to be directly responsible for the abrogation of cis-platin-induced reduction of tumor growth. PIFA were shown to activate a specific macrophage population in the red pulp of the spleen, with high F4/80 and low CD11b surface markers, which was necessary for the chemoresistance effect. This observation suggested that MSCs secreted PIFAs in response to cis-platin, which subsequently acted on splenic macrophages to trigger the release of other mediators that would act systemically to allow tumor cells to grow in the presence of platinum-drugs.
The receptor involved in mediating the chemoresistance effect of 12-HHT was confirmed to be BLT2, the type-2 leukotriene B receptor, which is a known low-affinity receptor for 12-HHT. In contrast, the site of recognition for 16:4 n-3 had remained unclear. Interestingly, splenic macrophage activation by PIFAs led to the formation and secretion of several lysophospholipids, detected in the conditioned medium that could induce chemoresistance in the mouse model. The specific lysophospholipid that mediated chemoresistance had remained unidentified. Notably, non-genotoxic cancer drugs such as paclitaxel or sorafenib did not trigger the chemoresistance response, suggesting that PIFAs might be mediating an extrinsic chemoresistance response that is initiated by drugs that are genotoxic and/or cause cell-cycle arrest.
In another separate line of research that has expanded in recent years, it has been established that the omega-3 LCPUFA docosahexaenoic acid (DHA) activates a membrane receptor called GPR120. Whereas initially linked to macrophages and the mediation of the anti-inflammatory actions of dietary omega-3 LCPUFA in mice on a high-fat diet, the involvement of GPR120 is now known in several processes including the regulation of insulin homeostasis, thermogenesis, satiety and bone growth. GPR120 is found on cells in the gastrointestinal tract, adipose tissue, as well as on different immune cell types. The receptor also mediates the anti-inflammatory effects in adipose tissue in response to dietary eicosapentaenoic acid (EPA) in a model of diet-induced obesity in mice. GPR120 was recently found to function in the hypothalamus as well, the brain’s appetite control center, mediating energy homeostasis and reducing
hypothalamic inflammation following the intake of a high caloric diet. While GPR120 is being evaluated as a promising drug target, for example in type 2 diabetes, the original notion that GPR120 is a specific receptor for DHA appeared increasingly less tenable, since a variety of unsaturated fatty acids, including alpha-linolenic acid (ALA), have also been found to activate GPR120. Interestingly, all act as agonists in their free fatty acid form, hence GPR120 is also called free fatty acid receptor 4, a member of a family of receptor proteins that sense the levels of various types of free fatty acids in tissues.
 In the present study, the same group of researchers that had uncovered the PIFAs tested the possibility that 16:4 n-3 might activate splenic macrophages by binding to GPR120, or to another receptor for free fatty acids, GPR40 (free fatty acid receptor 1). Houthuijzen and colleagues first determined whether 16:4 n-3 could activate these receptors by employing a macrophage cell line in which the expression of these receptors could be induced. 16:4 n-3 activated both receptors, and did so more potently than ALA. Both GPR120 and GPR40 were confirmed to be expressed nearly exclusively by the F4/80 high/CD11b low macrophage cell type in the spleen.
In the present study, the same group of researchers that had uncovered the PIFAs tested the possibility that 16:4 n-3 might activate splenic macrophages by binding to GPR120, or to another receptor for free fatty acids, GPR40 (free fatty acid receptor 1). Houthuijzen and colleagues first determined whether 16:4 n-3 could activate these receptors by employing a macrophage cell line in which the expression of these receptors could be induced. 16:4 n-3 activated both receptors, and did so more potently than ALA. Both GPR120 and GPR40 were confirmed to be expressed nearly exclusively by the F4/80 high/CD11b low macrophage cell type in the spleen.
In a functional assay, the intravenous administration of the cell culture medium of mouse spleen cells stimulated with 16:4 n-3 produced chemoresistance to cis-platin in the murine tumor model. Furthermore, synthetic agonists of GPR120 and GPR40 were able to do this, indicating that both GPR120 and GPR40 could be involved in tumor cell growth and the overcoming of the inhibition of tumor growth in mice induced by cis-platin. The chemoresistance effect initiated by exposure of macrophages to 16:4 n-3 could, however, only be inhibited by an antagonist of GPR120, but not by blocking GPR40. Additionally, the chemoresistance imparted by 16:4 n-3 was confirmed using mice that had the GPR120 receptor genetically “knocked-out.” The results strongly suggest that some factor that renders tumor cells resistant to the antineoplastic activity of cis-platin can be secreted by spleen cells upon stimulation by 16:4 n-3 through the activation of GPR120.
The researchers had already determined that 16:4 n-3 was able to trigger the formation of several lysophospholipid species by spleen macrophages. The production of lysophospholipids requires the involvement of a phospholipase, an enzyme that cleaves one of the two fatty acid moieties from a phospholipid. In the present study, the involvement of a cytosolic phospholipase A2 (cPLA2) in the chemoresistance response to 16:4 n-3 was confirmed. Furthermore, among three lysophospholipids found in the conditioned medium of spleen cells exposed to 16:4 n-3, only one was reduced by an inhibitor of this cPLA2. This lysophospholipid, nervonoyl-lysophosphatidylcholine (LPC(24:1)) is rather unusual because it contains nervonic acid (C24:1), a long-chain monounsaturated fatty acid that is present in tissues at relatively low levels, with very little known about its physiological function. The treatment of tumor-bearing mice that had their spleens removed with LPC(24:1) did induce chemoresistance to cis-platin. A structurally similar lysophospholipid, LPC(24:0) did not have this effect, suggesting a tight structural requirement to induce resistance of tumors to platinum-based chemotherapy.
The study next made an effort to translate the fundamental insight obtained in mice to the human situation, by confirming that human spleens contain a very similar immunologically-defined macrophage population that also expresses GPR120. Initiating the treatment of cancer patients with platinum-containing chemotherapeutics increased the levels of LPC(24:1) by about 25% in plasma four hours after treatment onset. This was not observed in patients placed on treatment with non-platinum-based chemotherapeutics. Although the increase was not statistically significant, the preliminary results are concordant with the possibility that a chemoresistance response to cis-platin in humans may be mediated by the formation of LPC(24:1) in the spleen.
 This study provides remarkable new insight in the mechanism of extrinsic tumor chemoresistance to the widely used chemotherapeutic substance cis-platin. The loss of sensitivity of tumors to cis-platin appears complex, involving several cell types and organs mediating a cascade of events at a systemic level, and involves “new” lipids that had not been implicated previously in other biological functions. Future research will need to confirm these findings, and further establish this pathway in the human setting. For the treatment of cancer patients, GPR120 may now be monitored more closely as a receptor that, when blocked, may render tumor cells sensitive to alkylating or genotoxic chemotherapeutic agents. Further research addressing the role of individual omega-3 PUFA such as 16:4 n-3, ALA, EPA and DHA, as endogenous ligands for GPR120, and their role in the modulation of replication stress or genotoxicity will be of high interest.
This study provides remarkable new insight in the mechanism of extrinsic tumor chemoresistance to the widely used chemotherapeutic substance cis-platin. The loss of sensitivity of tumors to cis-platin appears complex, involving several cell types and organs mediating a cascade of events at a systemic level, and involves “new” lipids that had not been implicated previously in other biological functions. Future research will need to confirm these findings, and further establish this pathway in the human setting. For the treatment of cancer patients, GPR120 may now be monitored more closely as a receptor that, when blocked, may render tumor cells sensitive to alkylating or genotoxic chemotherapeutic agents. Further research addressing the role of individual omega-3 PUFA such as 16:4 n-3, ALA, EPA and DHA, as endogenous ligands for GPR120, and their role in the modulation of replication stress or genotoxicity will be of high interest.
Future studies may address how 16:4 n-3, as a minor and relatively unknown polyunsaturated fatty acid, can have such a marked immunoregulatory effect, and whether its involvement is broader than that seen in the context of malignant cell growth alone. Recent advances in tumor biology research have shown that neoplastic cells have phenotypic plasticity, allowing dedifferentiation into stem cells with invasive and self-renewing properties, and redifferentiation into more differentiated cells. This has also offered a new interpretation of cancer development, incorporating the concept of clonal expansion of mutant stem cells. The dedifferentiation into cancer stem cells, also called the epithelial-mesenchymal transition, has recently been documented to have particular importance in the metastasis (dissemination of tumor cells to other organs) of chemoresistant cancer cells. Whether cancer stem cells with mesenchymal cell-like properties within tumors are the principal sites of 16:4 n-3 production, or normal mesenchymal stem cells in other organs also employ PIFA production as a physiological response to genotoxic substance exposure, remains to be studied.
 The relative impact of dietary 16:4 n-3 as a minor fatty acid affecting susceptibility to chemotherapy has not been systematically addressed. This fatty acid is biosynthesized by green microalgae for algal galactolipid formation, and all animals in the food chain that rely on preformed marine algae-derived PUFA in their diets are expected to contain low levels of 16:4 n-3 in their tissues. The description of 16:4 n-3 as a mediator that can act at a distinct site of action in its free fatty acid form to activate splenic macrophages further contributes to the idea that PUFA have signalling functions as free fatty acids, as was initially suggested for DHA acting on GPR120 to regulate macrophage inflammatory phenotype. The relative contribution of 16:4 n-3 to endogenous GPR120 activation compared with other much more abundant omega-3 LCPUFA, such as EPA and DHA, has however not been established. The pharmacology of Free Fatty Acid receptors is complex: not all ligands act as full active-site agonists, and the agonist selectivity of GPR120 is permissive with several PUFA of both the omega-3 and the omega-6 families being described to function as agonists at the receptor, including the relatively abundant fatty acid linoleic acid. A recent study has also cast doubt on GPR120 being uniquely involved in mediating the anti-inflammatory effect of dietary DHA, in the context of a high fat intake. Furthermore, dietary DHA has recently been found to increase the expression of GPR120 in mice. With the discovery of 16:4 n-3 as a ligand for GPR120, new research on the importance of this hitherto overlooked omega-3 PUFA of low abundance will gather interest. The selective involvement of 16:4 n-3 as a mediator of a potentially protective response of stem cells to genotoxic agents will be interesting to explore further, and this may lead to improvements in cancer treatment.
The relative impact of dietary 16:4 n-3 as a minor fatty acid affecting susceptibility to chemotherapy has not been systematically addressed. This fatty acid is biosynthesized by green microalgae for algal galactolipid formation, and all animals in the food chain that rely on preformed marine algae-derived PUFA in their diets are expected to contain low levels of 16:4 n-3 in their tissues. The description of 16:4 n-3 as a mediator that can act at a distinct site of action in its free fatty acid form to activate splenic macrophages further contributes to the idea that PUFA have signalling functions as free fatty acids, as was initially suggested for DHA acting on GPR120 to regulate macrophage inflammatory phenotype. The relative contribution of 16:4 n-3 to endogenous GPR120 activation compared with other much more abundant omega-3 LCPUFA, such as EPA and DHA, has however not been established. The pharmacology of Free Fatty Acid receptors is complex: not all ligands act as full active-site agonists, and the agonist selectivity of GPR120 is permissive with several PUFA of both the omega-3 and the omega-6 families being described to function as agonists at the receptor, including the relatively abundant fatty acid linoleic acid. A recent study has also cast doubt on GPR120 being uniquely involved in mediating the anti-inflammatory effect of dietary DHA, in the context of a high fat intake. Furthermore, dietary DHA has recently been found to increase the expression of GPR120 in mice. With the discovery of 16:4 n-3 as a ligand for GPR120, new research on the importance of this hitherto overlooked omega-3 PUFA of low abundance will gather interest. The selective involvement of 16:4 n-3 as a mediator of a potentially protective response of stem cells to genotoxic agents will be interesting to explore further, and this may lead to improvements in cancer treatment.
Houthuijzen JM, Oosterom I, Hudson BD, Hirasawa A, Daenen LGM, McLean CM, Hansen SVF, van Jaarsveld MTM, Peeper DS, Jafari Sadatmand S, Roodhart JML, van de Lest CHA, Ulven T, Ishihara K, Milligan G, Voest EE. Fatty acid 16:4(n-3) stimulates a GPR120-induced signaling cascade in splenic macrophages to promote chemotherapy resistance. FASEB J. 2017;31(5):2195-2209. [PubMed]
Worth Noting
Degraeve-Guilbault C, Bréhélin C, Haslam RP, Sayanova O, Marie-Luce G, Jouhet J, Corellou F. Glycerolipidome peculiarities of the smallest green picoeukaryote Ostreococcus tauri. Plant Physiol. 2017, Feb. [PubMed]
Dragano NRV, Solon C, Ramalho AF, de Moura RF, Razolli DS, Christiansen E, Azevedo C, Ulven T, Velloso LA. Polyunsaturated fatty acid receptors, GPR40 and GPR120, are expressed in the hypothalamus and control energy homeostasis and inflammation. J. Neuroinflammation 2017;14(91):1-16. [PubMed]
Fischer KR, Durrans A, Lee S, Sheng J, Li F, Wong ST, Choi H, El Rayes T, Ryu S, Troeger J, Schwabe RF, Vahdat LT, Altorki NK, Mittal V, Gao D. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature 2015;527(7579):472-476. [PubMed]
Galluzzi L, Vitale I, Michels J, Brenner C, Szabadkai G, Harel-Bellan A, Castedo M, Kroemer G. Systems biology of cisplatin resistance: past, present and future. Cell Death Dis. 2014;5:e1257. [PubMed]
Houthuijzen JM, Daenen LG, Roodhart JM, Oosterom I, van Jaarsveld MT, Govaert KM, Smith ME, Sadatmand SJ, Rosing H, Kruse F, Helms BJ, van Rooijen N, Beijnen JH, Haribabu B, van de Lest CH, Voest EE. Lysophospholipids secreted by splenic macrophages induce chemotherapy resistance via interference with the DNA damage response. Nat. Commun. 2014;5(5275):1-10. [PubMed]
Kim JM, Lee KP, Park SJ, Kang S, Huang J, Lee JM, Sato K, Chung HY, Okajima F, Im DS. Omega-3 fatty acids induce Ca(2+) mobilization responses in human colon epithelial cell lines endogenously expressing FFA4. Acta Pharmacol. Sin. 2015;36(7):813-820. [PubMed]
Liu M, Saeki K, Matsunobu T, Okuno T, Koga T, Sugimoto Y, Yokoyama C, Nakamizo S, Kabashima K, Narumiya S, Shimizu T, Yokomizo T. 12-Hydroxyheptadecatrienoic acid promotes epidermal wound healing by accelerating keratinocyte migration via the BLT2 receptor. J. Exp. Med. 2014;211(6):1063-1078. [PubMed]
Milligan G, Shimpukade B, Ulven T, Hudson BD. Complex pharmacology of Free Fatty Acid Receptors. Chem. Rev. 2017;117(1):67-110. [PubMed]
Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 2010;142(5):687-698. [PubMed]
Paerregaard SI, Agerholm M, Serup AK, Ma T, Kiens B, Madsen L, Kristiansen K, Jensen BA. FFAR4 (GPR120) signaling is not required for anti-inflammatory and insulin-sensitizing effects of omega-3 fatty acids. Mediators Inflamm. 2016;2016(1536047):1-12. [PubMed]
Quesada-López T, Cereijo R, Turatsinze JV, Planavila A, Cairó M, Gavaldà-Navarro A, Peyrou M, Moure R, Iglesias R, Giralt M, Eizirik DL, Villarroya F. The lipid sensor GPR120 promotes brown fat activation and FGF21 release from adipocytes. Nat. Commun. 2016;7(13479):1-17. [PubMed]
Roodhart JM, Daenen LG, Stigter EC, Prins HJ, Gerrits J, Houthuijzen JM, Gerritsen MG, Schipper HS, Backer MJ, van Amersfoort M, Vermaat JS, Moerer P, Ishihara K, Kalkhoven E, Beijnen JH, Derksen PW, Medema RH, Martens AC, Brenkman AB, Voest EE. Mesenchymal stem cells induce resistance to chemotherapy through the release of platinum-induced fatty acids. Cancer Cell 2011;20(3):370-383. [PubMed]
Scheel C, Weinberg RA. Phenotypic plasticity and epithelial-mesenchymal transitions in cancer and normal stem cells? Int. J. Cancer 2011;129(10):2310-2314. [PubMed]
Yamada H, Umemoto T, Kakei M, Momomura SI, Kawakami M, Ishikawa SE, Hara K. Eicosapentaenoic acid shows anti-inflammatory effect via GPR120 in 3T3-L1 adipocytes and attenuates adipose tissue inflammation in diet-induced obese mice. Nutr. Metab. (Lond) 2017;14(33):1-11. [PubMed]
Zhao J, Wang H, Shi P, Wang W, Sun Y. Oncotarget. GPR120, a potential therapeutic target for experimental colitis in IL-10 deficient mice. 2017;8(5):8397-8405. [PubMed]

