Modulation of the Acute Phase Response by Polyunsaturated Fatty Acids in Older People
This article at a glance
- Two recently published studies report on the relations between dietary intake and levels of poly-unsaturated fatty acids (PUFA) in blood and the acute phase response C-reactive protein (CRP) in older people.
- In one study, the omega-3 index was negatively associated with the levels of CRP and several other circulating markers of inflammation.
- In a second study, higher intake of omega-6 PUFA, and total PUFA, but not omega-3 PUFA, were found to be negatively associated with CRP, particularly in older women.
- The results reflect current challenges in understanding the roles of PUFA families in inflammation and the acute phase response.
 The acute phase response is a systemic reaction of the body to control inflammation that follows a local tissue injury. An acute-phase response involves fever, leukocytosis, release of cortisol, activation of complement and clotting cascades, changes in metal homeostasis, and a marked increase in the concentration of certain plasma proteins of hepatic origin. The latter hepatic acute phase response is initiated via the release of interleukin-6 (IL-6) from monocytes and macrophages that are activated during the course of an inflammatory response. IL-6 activates the secretion of a range of proteins by the liver into the circulation, each having specific roles in host defense, microbial clearance, and organ functioning and healing in the face of trauma and infection. Additional pro-inflammatory factors, such as the cytokines IL-1 and tumor necrosis factor-α, that are secreted into the circulation by an inflamed tissue also take part in fine-tuning the hepatic acute phase response. Kupffer cells, macrophages located in the liver, sense infection in blood and form additional cytokines that stimulate acute protein formation and secretion. Whereas the local inflammatory response involves its own mechanisms of onset and its return to homeostasis, the acute phase response is a systemic reaction that initially helps control infections and limit trauma, while at later stages it promotes the resolution of system-wide aspects of inflammation such as fever, normalizes hormonal adaptations, and the release of mediators that promote tissue healing.
The acute phase response is a systemic reaction of the body to control inflammation that follows a local tissue injury. An acute-phase response involves fever, leukocytosis, release of cortisol, activation of complement and clotting cascades, changes in metal homeostasis, and a marked increase in the concentration of certain plasma proteins of hepatic origin. The latter hepatic acute phase response is initiated via the release of interleukin-6 (IL-6) from monocytes and macrophages that are activated during the course of an inflammatory response. IL-6 activates the secretion of a range of proteins by the liver into the circulation, each having specific roles in host defense, microbial clearance, and organ functioning and healing in the face of trauma and infection. Additional pro-inflammatory factors, such as the cytokines IL-1 and tumor necrosis factor-α, that are secreted into the circulation by an inflamed tissue also take part in fine-tuning the hepatic acute phase response. Kupffer cells, macrophages located in the liver, sense infection in blood and form additional cytokines that stimulate acute protein formation and secretion. Whereas the local inflammatory response involves its own mechanisms of onset and its return to homeostasis, the acute phase response is a systemic reaction that initially helps control infections and limit trauma, while at later stages it promotes the resolution of system-wide aspects of inflammation such as fever, normalizes hormonal adaptations, and the release of mediators that promote tissue healing.
Although the term “acute” implies a fast response, the appearance of acute phase proteins in the circulation can take several hours, and in the case of a chronic inflammatory disease, elevations in the concentrations of acute phase proteins can be found in blood over long periods of time. Disturbances in the acute phase response are involved in pathophysiological conditions such as the systemic inflammatory response syndrome. Since individual acute phase proteins play important roles in both supporting and controlling inflammatory reactions, their levels in blood constitute diagnostic and prognostic biomarkers in patients, for infection and inflammation. One of the acute phase proteins, C-reactive protein (CRP), has found widespread use as one such biomarker of inflammation. It is a “distal” marker though, i.e. an acute phase protein that is induced by pro-inflammatory cytokines.
The levels of CRP acute phase protein in the circulation can increase up to 10,000 fold during injury, involving both IL-6 and IL-1 for optimal hepatic synthesis and secretion. Originally recognized for its ability to bind to the phosphocholine part of teichoic acid of the pneumococcal C-polysaccharide cell wall (“C-reactivity”), CRP plays an important role in the opsonization of microbes. Complement activation targets CRP-opsonized microbes for phagocytic removal. CRP also plays a role in regulating local inflammatory responses, modulates angiogenesis and platelet formation, and can promote the non-phlogistic clearance of apoptotic cells by phagocytosis, an important event in turning off inflammation. An increase in CRP level is not specific for some particular disease, but correlates well with a range of chronic inflammatory disorders in humans. CRP-mediated complement activation is believed to be involved in the pathogenesis of a number of chronic inflammatory diseases, such as atherosclerosis, cardiomyopathy, Alzheimer’s disease, and rheumatoid arthritis. The measurement of CRP levels has thus found widespread predictive utility for its association with chronic disease risk.
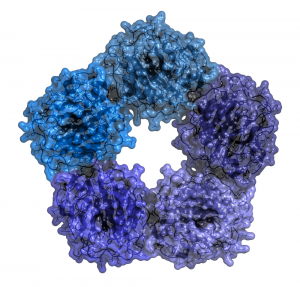 CRP circulates as a soluble pentameric structure in blood that can recognize infectious microbes or oxidatively-damaged cells. The active form of CRP is a monomer that is formed when CRP binds to its target structures (microbial and oxidized lipid epitopes). Current research is increasingly focussed on how pharmacological intervention can mitigate the involvement of CRP in chronic inflammatory pathologies, either through reducing elevated levels seen in chronic inflammatory disease, interfering with the transition from CRP pentamers to monomers, or by inhibition of CRP-mediated complement activation. Successful intervention to readjust a disturbed acute phase response may also need to look beyond one acute phase protein alone, as the acute phase response involves upregulation of specific proteins (positive acute phase reactants) and downregulation of others (negative acute phase reactants) during inflammatory reactions. However, results from recent genetic studies are not supportive in assigning a causal involvement of elevated CRP levels in cardiovascular disease, casting doubt on a direct role of the acute phase response in the etiology of chronic inflammatory disorders.
CRP circulates as a soluble pentameric structure in blood that can recognize infectious microbes or oxidatively-damaged cells. The active form of CRP is a monomer that is formed when CRP binds to its target structures (microbial and oxidized lipid epitopes). Current research is increasingly focussed on how pharmacological intervention can mitigate the involvement of CRP in chronic inflammatory pathologies, either through reducing elevated levels seen in chronic inflammatory disease, interfering with the transition from CRP pentamers to monomers, or by inhibition of CRP-mediated complement activation. Successful intervention to readjust a disturbed acute phase response may also need to look beyond one acute phase protein alone, as the acute phase response involves upregulation of specific proteins (positive acute phase reactants) and downregulation of others (negative acute phase reactants) during inflammatory reactions. However, results from recent genetic studies are not supportive in assigning a causal involvement of elevated CRP levels in cardiovascular disease, casting doubt on a direct role of the acute phase response in the etiology of chronic inflammatory disorders.
Omega-3 LCPUFA have long been recognized to be involved in regulating inflammatory reactions, and both enhanced intake as well as higher blood levels are associated with lower levels of positive acute phase reactants, and decreased tissue inflammation. Omega-6 LCPUFA can also serve anti-inflammatory roles in the context of inflammation, since arachidonic acid is the substrate for the formation of several anti-inflammatory mediators that regulate the extent and duration of inflammatory reactions. For example, a negative association between plasma concentrations of arachidonic acid and IL-6, and a positive association with anti-inflammatory transforming growth factor-β,have been reported. On the other hand, intake of omega-6 PUFA, in particular linoleic acid, in significant excess compared to levels that cover the essential requirement, may contribute to chronic inflammatory disorders typical of a Westernized lifestyle, particularly with a low dietary intake of omega-3 PUFA. On the background of this awareness, two recent prospective studies carried out in relatively large population-based cohorts assessed the association of dietary and blood fatty acids, with the levels of CRP, and other markers of systemic inflammation.
The first study aimed to determine the association of dietary intake of total PUFA, omega-6 PUFA and omega-3 PUFA, with CRP levels in adults aged 55 years or older. Data were collected over two periods during the 1990s in participants of the Rotterdam Study by Muka and colleagues at the Department of Epidemiology, Erasmus University Medical Center, in Rotterdam, The Netherlands. The Rotterdam Study is a prospective cohort study that has been ongoing since 1989 in the city of Rotterdam and has provided epidemiological insight in multiple disease endpoints. A large group of people (nearly 3000 individuals) was followed over a 6-8 year period. The study examined the influence of a large number of potential confounders and used separate analysis by gender. At baseline (1990-1993), study participants had been extensively interviewed at home by trained personnel about their lifestyle and health status. A clinical examination was subsequently performed at the study center. Dietary intake of different foods was assessed via a validated food-frequency questionnaire at baseline, allowing the recording of monthly food item intake during a preceding one-year period. Total energy intake and fatty acid intake was calculated from the dietary intake data using a food composition table. CRP was measured in serum samples obtained during the first and a third examination cycle that was organized approximately seven years later (1997-1999). Of all participants that had been evaluated in the first study period, a CRP measurement was available together with a dietary intake assessment for 2911 persons.
The mean age of the study participants was ~67 years at baseline. Total PUFA intake was 18.1 g/d in men, and 13.6 g/d in women. Omega-6 PUFA and omega-3 PUFA intake was 14.7 g/d and 1.2 g/d in men, and 11.1 and 0.98 g/d in women, respectively. The women had a comparatively higher level of education than the men. Nearly 47% of the men and 30% of the women had some prevalent chronic disease. Dietary intake of fish was relatively low: 15 g/d in men and 14 g/d in women. They had a relatively high intake of butter, margarine and hard frying fats (38.2 g/d in men and 26.46 g/d in women). At baseline, intake of vegetable oil, butter, margarine and hard frying fats, and whole grains correlated best with omega-6 PUFA intake. Butter, margarines and hard frying fats, fish, and red and processed meat intakes correlated best with omega-3 PUFA intake. The reason why meat as well as butter, margarine and frying fats correlated with omega-3 intake is because of the use of alpha-linolenic acid (ALA)-containing vegetable oils in the cooking/frying of meats, and this fatty acid being present in widely used margarine and sauces.
Mean CRP levels in the first study period were 0.187 mg/dl in men, and 0.176 mg/dl in women. These levels had risen to 0.235 and 0.241 mg/dl, respectively, in the third study period (1997-1999). Analyses examined dietary fatty acid intake as categorical variables organized in quartiles of energy-adjusted PUFA intake against CRP levels. The median CRP level was significantly lower as total PUFA intake or omega-6 PUFA intake increased. These associations remained statistically significant after adjustment for a range of covariates (such as whether people had some form of chronic disease, were complying or not with the recommended healthy diet standards, took anti-inflammatory drugs, their physical activity, educational level and household income). In contrast, there was no consistent association between omega-3 PUFA intake or the omega-3 to omega-6 PUFA intake ratio and CRP levels.
 A further analysis addressed whether the relationship between PUFA intake and CRP levels was different between women and men. That proved to be the case; in women a clear trend in higher total PUFA intake or higher omega-6 intake was associated with lower CRP levels. The ratio of omega-3 to omega-6 intake (but not omega-3 PUFA intake alone) showed a positive association with CRP levels in women. There were no significant associations between CRP levels and any of the studied fatty acid intake variables in men. In this older Dutch population, CRP levels in women were negatively associated with the amount of PUFA in their diet, in particular omega-6 PUFA intake. The associations between intake of individual fatty acids of omega-6 and omega-3 families with CRP levels were not determined. The results suggest that higher dietary PUFA intake, in particular omega-6 PUFA, is associated with a less intense acute phase response, and more notably in older women.
A further analysis addressed whether the relationship between PUFA intake and CRP levels was different between women and men. That proved to be the case; in women a clear trend in higher total PUFA intake or higher omega-6 intake was associated with lower CRP levels. The ratio of omega-3 to omega-6 intake (but not omega-3 PUFA intake alone) showed a positive association with CRP levels in women. There were no significant associations between CRP levels and any of the studied fatty acid intake variables in men. In this older Dutch population, CRP levels in women were negatively associated with the amount of PUFA in their diet, in particular omega-6 PUFA intake. The associations between intake of individual fatty acids of omega-6 and omega-3 families with CRP levels were not determined. The results suggest that higher dietary PUFA intake, in particular omega-6 PUFA, is associated with a less intense acute phase response, and more notably in older women.
In the second study, Fontes and colleagues investigated the relationship between red blood cell fatty acid composition and CRP in older people. The study was carried out by members of research groups in the Schools of Medicine, and Public Health at Boston University MA, and the Sanford School of Medicine, University of South Dakota, coordinated by the National Heart Lung and Blood Institute and Boston University’s Framingham Heart Study, Framingham, MA. Participants from two cohorts of the Framingham Heart Study, the Framingham Offspring Cohort (recruited in 1970) and the Framingham Omni Cohort (participants with a racially-diverse background, recruited in 1994), were evaluated in their eighth and third examination cycles, respectively. The average age of the participants determined at clinical examination was 66 years. Of 2724 subjects in the combined cohorts the fatty acid composition of red blood cells (RBC), levels of a range of plasma and urine inflammatory biomarkers, and measurement of several clinical covariates was available. RBC fatty acid measurements were made after methylation of RBCs followed by gas chromatographic detection. The omega-3 index was calculated as the percentage of EPA plus DHA of total fatty acids. In addition to CRP and IL-6, eight biomarkers of inflammation were measured.
 Prevalent cardiovascular disease was relatively low in the participants (6%), but nearly half received blood-pressure lowering or lipid-lowering drugs, and took aspirin at least three times a week. About one in ten persons used fish oil supplements. The subjects had a lipid profile within the normal range, but were slightly overweight on average. All measured inflammatory markers, including CRP and IL-6, were negatively correlated with the omega-3 index after adjustment for age and sex. After further adjustment for a range of potential confounders, all correlations remained statistically significant, except for osteoprotegerin and MCP-1. The associations for IL-6, 8-epi-PGF2α (a marker for non-enzymatic oxidation and rearrangement of AA), and lipoprotein phospholipase A2, were strongest.
Prevalent cardiovascular disease was relatively low in the participants (6%), but nearly half received blood-pressure lowering or lipid-lowering drugs, and took aspirin at least three times a week. About one in ten persons used fish oil supplements. The subjects had a lipid profile within the normal range, but were slightly overweight on average. All measured inflammatory markers, including CRP and IL-6, were negatively correlated with the omega-3 index after adjustment for age and sex. After further adjustment for a range of potential confounders, all correlations remained statistically significant, except for osteoprotegerin and MCP-1. The associations for IL-6, 8-epi-PGF2α (a marker for non-enzymatic oxidation and rearrangement of AA), and lipoprotein phospholipase A2, were strongest.
The two studies had a comparable size and age range of participants. The results show CRP levels associated with total PUFA and omega-6 PUFA intake, and omega-3 PUFA EPA and DHA levels in RBC membranes. The study by Muka and colleagues offers appreciation for a potential association of omega-6 PUFA intake with the acute phase response in older people. A much weaker support for an association with omega-3 PUFA intake is noted. The study by Fontes and colleagues shows EPA+DHA present in red blood cell membranes, as a marker of omega-3 LCPUFA, associated with a number of inflammatory biomarkers, suggesting perhaps that EPA and DHA can influence inflammation through multiple pathways. None of the associations should, however, be interpreted as causal relations.
In the study by Muka and colleagues a correlation between a higher intake of omega-6 PUFA and lower CRP levels may suggest that omega-6 PUFA reduce the acute phase response, for example by modifying the mechanisms by which CRP is formed and released by the liver. Alternatively, there may be effects on enhanced CRP turn-over, or anti-inflammatory actions associated with omega-6 PUFA intake, which provide a lower stimulus for hepatic CRP release. At present it is not possible to discriminate between such possibilities, but the observation that higher PUFA intake and particularly that of omega-6 PUFA intake are associated with a lower acute phase response is an important observation. The authors indicate that in the studied population cohort omega-3 intake is dominated by ALA. A conclusion that marine fish-derived omega-3 would not have an influence over CRP levels may be premature, since this population may have had a low omega-3 status and a low fish intake.
 The results of the Muka study are not completely in line with associations identified in a third prospective study (12 year follow-up) carried out in a large cohort of middle-age French individuals published by Julia et al in the British Journal of Nutrition in 2013. In that study, a dietary pattern reflecting a high omega-6 to omega-3 fatty acid intake ratio was positively associated with elevated CRP. That dietary pattern corresponded to a diet poor in fatty fish, seafood, and margarines, and rich in other animal products (meats and eggs). In a further analysis, total omega-3 PUFA, omega-3 LCPUFA, and omega-6 PUFA intake were each individually negatively associated with CRP levels, but the associations were modified by the dietary intake of vitamin E, suggesting that additional dietary factors may modify the associations. The studies of Muka and Fontes provide interesting data of associations between total and omega-6 PUFA intake, and RBC omega-3 PUFA with lower CRP levels. These data however are in contrast with randomized controlled trials that have shown for example that omega-3 LCPUFAs do not affect CRP levels. Results obtained from population studies need to be considered in light of possible effects of unmeasured confounders such as demonstrated by Julia et al.
The results of the Muka study are not completely in line with associations identified in a third prospective study (12 year follow-up) carried out in a large cohort of middle-age French individuals published by Julia et al in the British Journal of Nutrition in 2013. In that study, a dietary pattern reflecting a high omega-6 to omega-3 fatty acid intake ratio was positively associated with elevated CRP. That dietary pattern corresponded to a diet poor in fatty fish, seafood, and margarines, and rich in other animal products (meats and eggs). In a further analysis, total omega-3 PUFA, omega-3 LCPUFA, and omega-6 PUFA intake were each individually negatively associated with CRP levels, but the associations were modified by the dietary intake of vitamin E, suggesting that additional dietary factors may modify the associations. The studies of Muka and Fontes provide interesting data of associations between total and omega-6 PUFA intake, and RBC omega-3 PUFA with lower CRP levels. These data however are in contrast with randomized controlled trials that have shown for example that omega-3 LCPUFAs do not affect CRP levels. Results obtained from population studies need to be considered in light of possible effects of unmeasured confounders such as demonstrated by Julia et al.
Future studies will likely increasingly focus on the specific contributions that individual PUFA species make to immune regulation, and further determine if PUFA status is helpful in making sense of how the biological system is modulated by its dietary habits. A recent example of further comprehension is the recognition that docosapentaenoic acid (n-3) abundancy in RBC membranes also displays an inverse association with CRP levels. Whether older people have a need for both a higher omega-6 and omega-3 intake to control inflammation will be an important topic to determine in the future. A better understanding of a biomarker’s biological functions, such as for CRP in the context of the acute phase response and in chronic inflammatory pathologies in older people, will also be helpful for interpreting what changes in its levels mean.
Muka T, Kiefte-de Jong JC, Hofman A, Dehghan A, Rivadeneira F, Franco OH. Polyunsaturated fatty acids and serum C-reactive protein: the Rotterdam study. Am. J. Epidemiol. 2015;181(11):846-856. [PubMed]
Fontes JD, Rahman F, Lacey S, Larson MG, Vasan RS, Benjamin EJ, Harris WS, Robins SJ. Red blood cell fatty acids and biomarkers of inflammation: a cross-sectional study in a community-based cohort. Atherosclerosis 2015;240(2):431-436. [PubMed]
Worth Noting
Agrawal A, Gang TB, Rusiñol AE. Recognition functions of pentameric C-reactive protein in cardiovascular disease. Mediators Inflamm. 2014;2014(319215):1-7. [PubMed]
Ahmed MS, Jadhav AB, Hassan A, Meng QH. Acute phase reactants as novel predictors of cardiovascular disease. ISRN Inflamm. 2012;2012:953461. [PubMed]
Du Clos TW. Pentraxins: structure, function, and role in inflammation. ISRN Inflamm. 2013;2013(379040):1-22. [PubMed]
Emerging Risk Factors Collaboration, Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, Danesh J. et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet 2010;375(9709):132-140. [PubMed]
Ferrucci L, Cherubini A, Bandinelli S, Bartali B, Corsi A, Lauretani F, Martin A, Andres-Lacueva C, Senin U, Guralnik JM. Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. J. Clin. Endocrinol. Metab. 2006;91(2):439-446. [PubMed]
Flock MR, Skulas-Ray AC, Harris WS, Gaugler TL, Fleming JA, Kris-Etherton PM. Effects of supplemental long-chain omega-3 fatty acids and erythrocyte membrane fatty acid content on circulating inflammatory markers in a randomized controlled trial of healthy adults. Prostaglandins Leukot. Essent. Fatty Acids 2014;91(4):161-168. [PubMed]
Gershov D, Kim S, Brot N, Elkon KB. C-Reactive protein binds to apoptotic cells, protects the cells from assembly of the terminal complement components, and sustains an antiinflammatory innate immune response: implications for systemic autoimmunity. J. Exp. Med. 2000;192(9):1353-1364. [PubMed]
Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem. J. 1990;265(3):621-636. [PubMed]
Hofman A, Brusselle GG, Darwish Murad S, van Duijn CM, Franco OH, Goedegebure A, Ikram MA, Klaver CC, Nijsten TE, Peeters RP, Stricker BH, Tiemeier HW, Uitterlinden AG, Vernooij MW. The Rotterdam Study: 2016 objectives and design update. Eur. J. Epidemiol. 2015;30(8):661-708. [PubMed]
Julia C, Meunier N, Touvier M, Ahluwalia N, Sapin V, Papet I, Cano N, Hercberg S, Galan P, Kesse-Guyot E. Dietary patterns and risk of elevated C-reactive protein concentrations 12 years later. Br. J. Nutr. 2013;110(4):747-754. [PubMed]
Julia C, Touvier M, Meunier N, Papet I, Galan P, Hercberg S, Kesse-Guyot E. Intakes of PUFAs were inversely associated with plasma C-reactive protein 12 years later in a middle-aged population with vitamin E intake as an effect modifier. J. Nutr. 2013;143(11):1760-1766. [PubMed]
Klipstein-Grobusch K, den Breeijen JH, Goldbohm RA, Geleijnse JM, Hofman A, Grobbee DE, Witteman JC. Dietary assessment in the elderly: validation of a semiquantitative food frequency questionnaire. Eur. J. Clin. Nutr. 1998;52(8):588-596. [PubMed]
Nijsten MWN, de Groot ER, ten Duis HJ, Klasen HJ, Hack CE, Aarden LA. Serum levels of interleukin-6 and acute phase responses. Lancet 1987;2(8564):921. [PubMed]
Nyström PO. The systemic inflammatory response syndrome: definitions and aetiology. J. Antimicrob. Chemother. 1998;41 (Suppl A:1-7):1-7. [PubMed]
Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008;233(6):674-688. [PubMed]
Skulas-Ray AC, Flock MR, Richter CK, Harris WS, West SG, Kris-Etherton PM. Red blood cell docosapentaenoic Acid (DPA n-3) is inversely associated with triglycerides and C-reactive protein (CRP) in healthy adults and dose-dependently increases following n-3 fatty acid supplementation. Nutrients 2015;7(8):6390-6404. [PubMed]
Strang F, Schunkert H. C-reactive protein and coronary heart disease: all said – is not it? Mediators Inflamm. 2014;2014:757123. [PubMed]
Wall R, Ross RP, Fitzgerald GF, Stanton C. Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr. Rev. 2010;68(5):280-289. [PubMed]
Weinhold B, Rüther U. Interleukin-6-dependent and -independent regulation of the human C-reactive protein gene. Biochem. J. 1997;327(2):425-429. [PubMed]
Zimmermann O, Li K, Zaczkiewicz M, Graf M, Liu Z, Torzewski J. C-reactive protein in human atherogenesis: facts and fiction. Mediators Inflamm. 2014;2014:561428. [PubMed]

