
Dry eye syndrome (dry eye) is one of the leading reasons for patient visits to ophthalmologists in part because its
symptoms are unpleasant. They include pain, dryness, grittiness, itchiness, burning, light sensitivity, difficulty reading, driving and doing computer work, all of which undermine one’s quality of life. Dry eye is a condition of insufficient tears to lubricate the eye and is common throughout the world. In the U.S., the condition affects about
3.3 and 1.7 million women and men over the age of 50 years, respectively, and 10 to 20% of adults
worldwide. It is even more prevalent in Asian populations, including
Japanese and
Chinese. Dry eye afflicts women more often than men and is associated with certain medications such as estrogen therapy, antidepressants, antihistamines and antihypertensives. It also
develops with increasing age, certain medical conditions, environmental exposures to smoke, wind and dry climate, extensive computer usage, the use of contact lenses and with eye surgeries, such as LASIK.

Dry eye syndrome is usually accompanied by the increased production of inflammatory cytokines in the
ocular surface cells and in
tears. These contribute to the symptoms of dry eye and the severity of the condition. Patients commonly use artificial tears to relieve symptoms. Depending on their formulation and period of use, artificial tears may not improve the ocular surface, reduce inflammation or affect the underlying
meibomian gland dysfunction. However, eye drops containing
anti-inflammatory agents have been associated with improved symptoms and reduced cytokines, although products with topical steroids may have serious
side effects. Eye drops containing
alpha-linolenic acid were reported to improve the symptoms of dry eye and significantly reduce the inflammatory mediators associated with the condition. One report described the effectiveness of topical treatment with
resolvin E1, a derivative of EPA, in reducing corneal staining by 80% and maintaining goblet cell density in a mouse model of dry eye syndrome. With these exceptions, the only studies on the effects of long-chain omega-3 PUFAs (n-3 LC-PUFAs) in dry eye disease provided these PUFAs via dietary intake. This study in an animal model reports the effect of n-3 LC-PUFAs furnished as a constituent of therapeutic eye drops. Dr Zhengri Li and colleagues at Chonnam National University, Gwangju, Korea, investigated the effectiveness of lubricant eyedrops containing one of two concentrations of n-3 LC-PUFAs with or without the addition of 0.1% hyaluronic acid (HLA) in dry eye syndrome. The study was performed using a
desiccating stress model of dry eye syndrome in mice treated systemically with scopolamine. Eye drops with
HLA have been associated with improved lissamine green staining and symptom frequency scores in patients with eye disease, but HLA not a component of all artificial tear products. The research objective was to assess eye disease severity using corneal irregularity scores assessed as
previously described and fluorescein staining. To assess inflammatory responses, the investigators measured the concentration of inflammatory cytokines and markers of oxidative stress in the conjunctiva in response to the condition alone and the varying treatments.

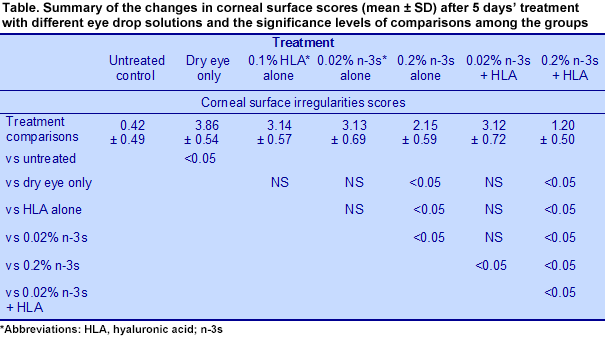
The experimental design included (1) untreated animals not exposed to dry eye conditions or topical treatment; (2) dry eye exposed animals without eye drops; (3) dry eye animals treated with 0.1% HLA drops; (4) dry eye animals treated with 0.02% n-3 LC-PUFAs alone; (5) dry eye animals treated with 0.2% n-3 LC-PUFAs; (6) dry eye animals treated with 0.02% n-3 LC-PUFAs and 0.1% HLA drops; (7) dry eye animals treated with 0.2% n-3 LC-PUFAs and 0.1% HLA drops. Eye drops (3μL) were given 4 times per day initiated at the induction of dry eye syndrome. Mice were evaluated for corneal irregularity scores ranked on a 6-point scale and fluorescein staining on a 4-point scale at 5 and 10 days after treatment. The animals were sacrificed 10 days after treatment began. The investigators measured the concentrations 3 inflammatory markers and 2 indicators of lipid peroxidation in conjunctiva tissue. These markers were interleukins (IL)-1β, IL-17 and interferon gamma-induced protein 10 (IP-10), hexanoyl-lys (HEL), a marker of acute lipid peroxidation, and 4-hydroxynonenal (4-HNE), a marker of chronic lipid peroxidation. The conjunctiva lines the eyelid and covers the white part of the eye. It contains epithelial and goblet cells. Five days after dry eye conditions were induced, corneal irregularity scores increased significantly in the dry eye animals compared with the untreated controls (3.86 ± 0.54 vs. 0.42 ± 0.49, P <0.05). The effects of the different treatments compared with the various groups are shown in the Table. Compared with the dry eye control animals, those treated with drops containing only HLA or 0.02% n-3 LC-PUFAs did not differ from the controls, even though their scores were lower. The animals treated with 0.2% n-3 LC-PUFAs alone had significantly lower corneal irregularity scores compared with the dry eye controls and treatments with only 0.1% HLA or the low-dose n-3 PUFAs. Overall, the eyedrops containing 0.2% n-3 LC-PUFAs plus HLA provided most effective treatment to improve corneal surface irregularities. Findings after 10 days of treatment were similar to those at 5 days.
Results from the corneal fluorescein staining, a test used to detect corneal damage and foreign bodies, followed a pattern similar to the corneal surface irregularities scores. The most effective treatment was the combination of 0.1% HLA and 0.2% n-3 LC-PUFAs, which significantly exceeded the improvements observed with HLA alone, 0.02% n-3 LC-PUFAs alone, 0.2% n-3 LC-PUFAs alone, or the combination of 0.02% n-3 LC-PUFAs with 0.1% HLA.

Conjunctival tissue cytokines increased significantly with the induction of dry eye syndrome, but were reduced the most with eyedrops containing 0.2% n-3 LC-PUFAs and HLA. IL-1β concentrations were significantly lower with drops having only HLA, 0.2% n-3 PUFAs, or 0.02% n-3 LC-PUFAs plus HLA, but the greatest suppression occurred with the 0.2% n-3 LC-PUFAs plus HLA treatment. In these animals, inflammatory markers were equivalent to the untreated animals without dry eye. Tissue levels of IL-17 and IP-10 were significantly lower with 0.2% n-3 LC-PUFAs alone, but the lowest concentrations were observed with the 0.2% n-3 LC-PUFAs plus HLA. The two markers of oxidative stress increased significantly with the induction of dry eye, but the concentrations of HEL were unaffected by any of the eye drop preparations. In contrast, the concentrations of 4-HNE were significantly reduced with drops containing 0.2% n-3 LC-PUFAs alone and further reduced when the drops contained both 0.2% n-3 LC-PUFAs and HLA. This study is the first demonstration of the effectiveness of topical n-3 LC-PUFAs in the alleviation of dry eye syndrome in an animal model. The investigators provided HLA and two concentrations of n-3 LC-PUFAs in artificial tear drops and observed the greatest reductions in corneal integrity and damage scores with drops containing 0.2% n-3 LC-PUFAs plus 0.1% HLA. In addition, 0.2% n-3 LC-PUFAs alone significantly lowered the concentrations of 3 inflammatory markers and a marker for chronic lipid peroxidation. These reductions were further improved by the addition of 0.1% HLA. This study, the report on resolvin E1 and the involvement of n-3 LC-PUFAs and their derivatives in corneal nerve regeneration as shown in animal studies, along with human studies on the dietary consumption of n-3 LC-PUFAs, all indicate that n-3 LC-PUFAs are potentially important in the treatment of dry eye syndrome. Li Z, Choi JH, Oh HJ, Park SH, Lee JB, Yoon KC. Effects of eye drops containing a mixture of omega-3 essential fatty acids and hyaluronic acid on the ocular surface in desiccating stress-induced murine dry eye.
Curr Eye Res 2014; Feb. 21. [
PubMed]
 Dry eye syndrome (dry eye) is one of the leading reasons for patient visits to ophthalmologists in part because its symptoms are unpleasant. They include pain, dryness, grittiness, itchiness, burning, light sensitivity, difficulty reading, driving and doing computer work, all of which undermine one’s quality of life. Dry eye is a condition of insufficient tears to lubricate the eye and is common throughout the world. In the U.S., the condition affects about 3.3 and 1.7 million women and men over the age of 50 years, respectively, and 10 to 20% of adults worldwide. It is even more prevalent in Asian populations, including Japanese and Chinese. Dry eye afflicts women more often than men and is associated with certain medications such as estrogen therapy, antidepressants, antihistamines and antihypertensives. It also develops with increasing age, certain medical conditions, environmental exposures to smoke, wind and dry climate, extensive computer usage, the use of contact lenses and with eye surgeries, such as LASIK.
Dry eye syndrome (dry eye) is one of the leading reasons for patient visits to ophthalmologists in part because its symptoms are unpleasant. They include pain, dryness, grittiness, itchiness, burning, light sensitivity, difficulty reading, driving and doing computer work, all of which undermine one’s quality of life. Dry eye is a condition of insufficient tears to lubricate the eye and is common throughout the world. In the U.S., the condition affects about 3.3 and 1.7 million women and men over the age of 50 years, respectively, and 10 to 20% of adults worldwide. It is even more prevalent in Asian populations, including Japanese and Chinese. Dry eye afflicts women more often than men and is associated with certain medications such as estrogen therapy, antidepressants, antihistamines and antihypertensives. It also develops with increasing age, certain medical conditions, environmental exposures to smoke, wind and dry climate, extensive computer usage, the use of contact lenses and with eye surgeries, such as LASIK.  Dry eye syndrome is usually accompanied by the increased production of inflammatory cytokines in the ocular surface cells and in tears. These contribute to the symptoms of dry eye and the severity of the condition. Patients commonly use artificial tears to relieve symptoms. Depending on their formulation and period of use, artificial tears may not improve the ocular surface, reduce inflammation or affect the underlying meibomian gland dysfunction. However, eye drops containing anti-inflammatory agents have been associated with improved symptoms and reduced cytokines, although products with topical steroids may have serious side effects. Eye drops containing alpha-linolenic acid were reported to improve the symptoms of dry eye and significantly reduce the inflammatory mediators associated with the condition. One report described the effectiveness of topical treatment with resolvin E1, a derivative of EPA, in reducing corneal staining by 80% and maintaining goblet cell density in a mouse model of dry eye syndrome. With these exceptions, the only studies on the effects of long-chain omega-3 PUFAs (n-3 LC-PUFAs) in dry eye disease provided these PUFAs via dietary intake. This study in an animal model reports the effect of n-3 LC-PUFAs furnished as a constituent of therapeutic eye drops. Dr Zhengri Li and colleagues at Chonnam National University, Gwangju, Korea, investigated the effectiveness of lubricant eyedrops containing one of two concentrations of n-3 LC-PUFAs with or without the addition of 0.1% hyaluronic acid (HLA) in dry eye syndrome. The study was performed using a desiccating stress model of dry eye syndrome in mice treated systemically with scopolamine. Eye drops with HLA have been associated with improved lissamine green staining and symptom frequency scores in patients with eye disease, but HLA not a component of all artificial tear products. The research objective was to assess eye disease severity using corneal irregularity scores assessed as previously described and fluorescein staining. To assess inflammatory responses, the investigators measured the concentration of inflammatory cytokines and markers of oxidative stress in the conjunctiva in response to the condition alone and the varying treatments.
Dry eye syndrome is usually accompanied by the increased production of inflammatory cytokines in the ocular surface cells and in tears. These contribute to the symptoms of dry eye and the severity of the condition. Patients commonly use artificial tears to relieve symptoms. Depending on their formulation and period of use, artificial tears may not improve the ocular surface, reduce inflammation or affect the underlying meibomian gland dysfunction. However, eye drops containing anti-inflammatory agents have been associated with improved symptoms and reduced cytokines, although products with topical steroids may have serious side effects. Eye drops containing alpha-linolenic acid were reported to improve the symptoms of dry eye and significantly reduce the inflammatory mediators associated with the condition. One report described the effectiveness of topical treatment with resolvin E1, a derivative of EPA, in reducing corneal staining by 80% and maintaining goblet cell density in a mouse model of dry eye syndrome. With these exceptions, the only studies on the effects of long-chain omega-3 PUFAs (n-3 LC-PUFAs) in dry eye disease provided these PUFAs via dietary intake. This study in an animal model reports the effect of n-3 LC-PUFAs furnished as a constituent of therapeutic eye drops. Dr Zhengri Li and colleagues at Chonnam National University, Gwangju, Korea, investigated the effectiveness of lubricant eyedrops containing one of two concentrations of n-3 LC-PUFAs with or without the addition of 0.1% hyaluronic acid (HLA) in dry eye syndrome. The study was performed using a desiccating stress model of dry eye syndrome in mice treated systemically with scopolamine. Eye drops with HLA have been associated with improved lissamine green staining and symptom frequency scores in patients with eye disease, but HLA not a component of all artificial tear products. The research objective was to assess eye disease severity using corneal irregularity scores assessed as previously described and fluorescein staining. To assess inflammatory responses, the investigators measured the concentration of inflammatory cytokines and markers of oxidative stress in the conjunctiva in response to the condition alone and the varying treatments.  The experimental design included (1) untreated animals not exposed to dry eye conditions or topical treatment; (2) dry eye exposed animals without eye drops; (3) dry eye animals treated with 0.1% HLA drops; (4) dry eye animals treated with 0.02% n-3 LC-PUFAs alone; (5) dry eye animals treated with 0.2% n-3 LC-PUFAs; (6) dry eye animals treated with 0.02% n-3 LC-PUFAs and 0.1% HLA drops; (7) dry eye animals treated with 0.2% n-3 LC-PUFAs and 0.1% HLA drops. Eye drops (3μL) were given 4 times per day initiated at the induction of dry eye syndrome. Mice were evaluated for corneal irregularity scores ranked on a 6-point scale and fluorescein staining on a 4-point scale at 5 and 10 days after treatment. The animals were sacrificed 10 days after treatment began. The investigators measured the concentrations 3 inflammatory markers and 2 indicators of lipid peroxidation in conjunctiva tissue. These markers were interleukins (IL)-1β, IL-17 and interferon gamma-induced protein 10 (IP-10), hexanoyl-lys (HEL), a marker of acute lipid peroxidation, and 4-hydroxynonenal (4-HNE), a marker of chronic lipid peroxidation. The conjunctiva lines the eyelid and covers the white part of the eye. It contains epithelial and goblet cells. Five days after dry eye conditions were induced, corneal irregularity scores increased significantly in the dry eye animals compared with the untreated controls (3.86 ± 0.54 vs. 0.42 ± 0.49, P <0.05). The effects of the different treatments compared with the various groups are shown in the Table. Compared with the dry eye control animals, those treated with drops containing only HLA or 0.02% n-3 LC-PUFAs did not differ from the controls, even though their scores were lower. The animals treated with 0.2% n-3 LC-PUFAs alone had significantly lower corneal irregularity scores compared with the dry eye controls and treatments with only 0.1% HLA or the low-dose n-3 PUFAs. Overall, the eyedrops containing 0.2% n-3 LC-PUFAs plus HLA provided most effective treatment to improve corneal surface irregularities. Findings after 10 days of treatment were similar to those at 5 days.
The experimental design included (1) untreated animals not exposed to dry eye conditions or topical treatment; (2) dry eye exposed animals without eye drops; (3) dry eye animals treated with 0.1% HLA drops; (4) dry eye animals treated with 0.02% n-3 LC-PUFAs alone; (5) dry eye animals treated with 0.2% n-3 LC-PUFAs; (6) dry eye animals treated with 0.02% n-3 LC-PUFAs and 0.1% HLA drops; (7) dry eye animals treated with 0.2% n-3 LC-PUFAs and 0.1% HLA drops. Eye drops (3μL) were given 4 times per day initiated at the induction of dry eye syndrome. Mice were evaluated for corneal irregularity scores ranked on a 6-point scale and fluorescein staining on a 4-point scale at 5 and 10 days after treatment. The animals were sacrificed 10 days after treatment began. The investigators measured the concentrations 3 inflammatory markers and 2 indicators of lipid peroxidation in conjunctiva tissue. These markers were interleukins (IL)-1β, IL-17 and interferon gamma-induced protein 10 (IP-10), hexanoyl-lys (HEL), a marker of acute lipid peroxidation, and 4-hydroxynonenal (4-HNE), a marker of chronic lipid peroxidation. The conjunctiva lines the eyelid and covers the white part of the eye. It contains epithelial and goblet cells. Five days after dry eye conditions were induced, corneal irregularity scores increased significantly in the dry eye animals compared with the untreated controls (3.86 ± 0.54 vs. 0.42 ± 0.49, P <0.05). The effects of the different treatments compared with the various groups are shown in the Table. Compared with the dry eye control animals, those treated with drops containing only HLA or 0.02% n-3 LC-PUFAs did not differ from the controls, even though their scores were lower. The animals treated with 0.2% n-3 LC-PUFAs alone had significantly lower corneal irregularity scores compared with the dry eye controls and treatments with only 0.1% HLA or the low-dose n-3 PUFAs. Overall, the eyedrops containing 0.2% n-3 LC-PUFAs plus HLA provided most effective treatment to improve corneal surface irregularities. Findings after 10 days of treatment were similar to those at 5 days.  Results from the corneal fluorescein staining, a test used to detect corneal damage and foreign bodies, followed a pattern similar to the corneal surface irregularities scores. The most effective treatment was the combination of 0.1% HLA and 0.2% n-3 LC-PUFAs, which significantly exceeded the improvements observed with HLA alone, 0.02% n-3 LC-PUFAs alone, 0.2% n-3 LC-PUFAs alone, or the combination of 0.02% n-3 LC-PUFAs with 0.1% HLA.
Results from the corneal fluorescein staining, a test used to detect corneal damage and foreign bodies, followed a pattern similar to the corneal surface irregularities scores. The most effective treatment was the combination of 0.1% HLA and 0.2% n-3 LC-PUFAs, which significantly exceeded the improvements observed with HLA alone, 0.02% n-3 LC-PUFAs alone, 0.2% n-3 LC-PUFAs alone, or the combination of 0.02% n-3 LC-PUFAs with 0.1% HLA.  Conjunctival tissue cytokines increased significantly with the induction of dry eye syndrome, but were reduced the most with eyedrops containing 0.2% n-3 LC-PUFAs and HLA. IL-1β concentrations were significantly lower with drops having only HLA, 0.2% n-3 PUFAs, or 0.02% n-3 LC-PUFAs plus HLA, but the greatest suppression occurred with the 0.2% n-3 LC-PUFAs plus HLA treatment. In these animals, inflammatory markers were equivalent to the untreated animals without dry eye. Tissue levels of IL-17 and IP-10 were significantly lower with 0.2% n-3 LC-PUFAs alone, but the lowest concentrations were observed with the 0.2% n-3 LC-PUFAs plus HLA. The two markers of oxidative stress increased significantly with the induction of dry eye, but the concentrations of HEL were unaffected by any of the eye drop preparations. In contrast, the concentrations of 4-HNE were significantly reduced with drops containing 0.2% n-3 LC-PUFAs alone and further reduced when the drops contained both 0.2% n-3 LC-PUFAs and HLA. This study is the first demonstration of the effectiveness of topical n-3 LC-PUFAs in the alleviation of dry eye syndrome in an animal model. The investigators provided HLA and two concentrations of n-3 LC-PUFAs in artificial tear drops and observed the greatest reductions in corneal integrity and damage scores with drops containing 0.2% n-3 LC-PUFAs plus 0.1% HLA. In addition, 0.2% n-3 LC-PUFAs alone significantly lowered the concentrations of 3 inflammatory markers and a marker for chronic lipid peroxidation. These reductions were further improved by the addition of 0.1% HLA. This study, the report on resolvin E1 and the involvement of n-3 LC-PUFAs and their derivatives in corneal nerve regeneration as shown in animal studies, along with human studies on the dietary consumption of n-3 LC-PUFAs, all indicate that n-3 LC-PUFAs are potentially important in the treatment of dry eye syndrome. Li Z, Choi JH, Oh HJ, Park SH, Lee JB, Yoon KC. Effects of eye drops containing a mixture of omega-3 essential fatty acids and hyaluronic acid on the ocular surface in desiccating stress-induced murine dry eye. Curr Eye Res 2014; Feb. 21. [PubMed]
Conjunctival tissue cytokines increased significantly with the induction of dry eye syndrome, but were reduced the most with eyedrops containing 0.2% n-3 LC-PUFAs and HLA. IL-1β concentrations were significantly lower with drops having only HLA, 0.2% n-3 PUFAs, or 0.02% n-3 LC-PUFAs plus HLA, but the greatest suppression occurred with the 0.2% n-3 LC-PUFAs plus HLA treatment. In these animals, inflammatory markers were equivalent to the untreated animals without dry eye. Tissue levels of IL-17 and IP-10 were significantly lower with 0.2% n-3 LC-PUFAs alone, but the lowest concentrations were observed with the 0.2% n-3 LC-PUFAs plus HLA. The two markers of oxidative stress increased significantly with the induction of dry eye, but the concentrations of HEL were unaffected by any of the eye drop preparations. In contrast, the concentrations of 4-HNE were significantly reduced with drops containing 0.2% n-3 LC-PUFAs alone and further reduced when the drops contained both 0.2% n-3 LC-PUFAs and HLA. This study is the first demonstration of the effectiveness of topical n-3 LC-PUFAs in the alleviation of dry eye syndrome in an animal model. The investigators provided HLA and two concentrations of n-3 LC-PUFAs in artificial tear drops and observed the greatest reductions in corneal integrity and damage scores with drops containing 0.2% n-3 LC-PUFAs plus 0.1% HLA. In addition, 0.2% n-3 LC-PUFAs alone significantly lowered the concentrations of 3 inflammatory markers and a marker for chronic lipid peroxidation. These reductions were further improved by the addition of 0.1% HLA. This study, the report on resolvin E1 and the involvement of n-3 LC-PUFAs and their derivatives in corneal nerve regeneration as shown in animal studies, along with human studies on the dietary consumption of n-3 LC-PUFAs, all indicate that n-3 LC-PUFAs are potentially important in the treatment of dry eye syndrome. Li Z, Choi JH, Oh HJ, Park SH, Lee JB, Yoon KC. Effects of eye drops containing a mixture of omega-3 essential fatty acids and hyaluronic acid on the ocular surface in desiccating stress-induced murine dry eye. Curr Eye Res 2014; Feb. 21. [PubMed]

